Abstract
Purpose
To investigate the optimal dual-time-point (DTP) approaches using dynamic 68Ga-PSMA-11 PET/CT imaging to generate parametric images for prostate cancer patients.
Methods
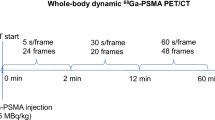
Fifteen patients with prostate cancer were intravenously administered 68Ga-PSMA-11 of 181.9 ± 47.2 MBq, followed by an immediate 60 min dynamic PET/CT scan. List-mode data were reconstructed into 25 timeframes (6 × 10 s, 8 × 30 s, and 11 × 300 s) and corrected for motion and partial volume effect. DTP parametric images were generated using different interval time points of 5 min and 10 min, with a minimum of 30 min time interval. Net influx rates (Ki) were calculated through the fitting of a single irreversible two-tissue compartmental model. Intraclass correlation coefficient (ICC) values between DTP protocols and 60 min Ki were obtained. Lesion-to-background ratios (LBRs) of Ki and standardized uptake value (SUV) images in each DTP protocol were determined.
Results
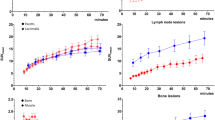
The DTP protocol of 5–10 min with a 40–45 min interval showed the highest ICC of 0.988 compared with the 60 min Ki, whereas the ICC values for the intervals of 0–5 min with 55–60 min and 0–10 min with 50–60 min were 0.941. The LBRs of the 60 min Ki, 5–10 min with 40–45 min Ki, 0–5 min with 55–60 min Ki, 0–10 min with 50–60 min Ki, SUVmean, and SUVmax images were 29.53 ± 27.33, 13.05 ± 15.28, 45.15 ± 53.11, 45.52 ± 70.31, 19.77 ± 23.43, and 25.06 ± 30.07, respectively.
Conclusion
The 0–5 min with 55–60 min DTP parametric imaging exhibits a comparable Ki to 60 min parametric imaging and remarkable image quality and contrast than SUV imaging, enhancing prostate cancer diagnosis while maintaining time efficiency.





Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics. CA Cancer J Clin. 2023;73:17–48.
Johansson JE, Holmberg L, Johansson S, Bergström R, Adami HO. Fifteen year survival in prostate cancer a prospective population-based study in Sweden. JAMA. 1997;277:467–71.
Pesapane F, Czarniecki M, Suter MB, Turkbey B, Villeirs G. Imaging of distant metastases of prostate cancer. Med Oncol. 2018;35:148.
Shou J, Zhang Q, Wang S, Zhang D. The prognosis of different distant metastases pattern in prostate cancer: a population based retrospective study. Prostate. 2018;78:491–7.
Haberkorn U, Eder M, Kopka K, Babich JW, Eisenhut M. New strategies in prostate cancer: prostate-specific membrane antigen (PSMA) ligands for diagnosis and therapy. Clin Cancer Res. 2016;22:9–15.
Chatachot K, Shiratori S, Chaiwatanarat T, Khamwan K. Patient dosimetry of 177Lu-PSMA I&T in metastatic prostate cancer treatment: the experience in Thailand. Ann Nucl Med. 2021;35:1193–202.
Grubmüller B, Baltzer P, D’Andrea D, Korn S, Haug AR, Hacker M, et al. 68Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy - diagnostic performance and impact on therapeutic decision-making. Eur J Nucl Med Mol Imaging. 2018;45:235–42.
Sonni I, Eiber M, Fendler WP, Alano RM, Vangala SS, Kishan AU, et al. Impact of 68Ga-PSMA-11 PET/CT on staging and management of prostate cancer patients in various clinical settings: a prospective single-center study. J Nucl Med. 2020;61:1153–60.
Hope TA, Aggarwal R, Chee B, Tao D, Greene KL, Cooperberg MR, et al. Impact of 68Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med. 2017;58:1956–61.
Fendler WP, Ferdinandus J, Czernin J, Eiber M, Flavell RR, Behr SC, et al. Impact of 68Ga-PSMA-11 PET on the management of recurrent prostate cancer in a prospective single-arm clinical trial. J Nucl Med. 2020;61:1793–9.
Ferraro DA, Garcia Schüler HI, Muehlematter UJ, Eberli D, Müller J, Müller A, et al. Impact of 68Ga-PSMA-11 PET staging on clinical decision-making in patients with intermediate or high-risk prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:652–64.
Khamwan K, Chamnan P, Thinka Y, Shiratori S. Determination of patient doses and biokinetic of 68Ga-PSMA-11 PET/CT imaging in metastases prostate cancer patients. J Med Imaging Radiat Sci. 2022;53:S62.
Bertoldo A, Rizzo G, Veronese M. Deriving physiological information from PET images: from SUV to compartmental modelling. Clin Transl Imaging. 2014;2:239–51.
Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50:11s–20s.
Dimitrakopoulou-Strauss A, Pan L, Sachpekidis C. Kinetic modeling and parametric imaging with dynamic PET for oncological applications: general considerations, current clinical applications, and future perspectives. Eur J Nucl Med Mol Imaging. 2021;48:21–39.
Ringheim A, Campos Neto GC, Anazodo U, Cui L, da Cunha ML, Vitor T, et al. Kinetic modeling of 68Ga-PSMA-11 and validation of simplified methods for quantification in primary prostate cancer patients. EJNMMI Res. 2020;10:12.
Wang G, Rahmim A, Gunn RN. PET parametric imaging: past, present, and future. IEEE Trans Radiat Plasma Med Sci. 2020;4:663–75.
Sachpekidis C, Pan L, Hadaschik BA, Kopka K, Haberkorn U, Dimitrakopoulou-Strauss A. 68Ga-PSMA-11 PET/CT in prostate cancer local recurrence: impact of early images and parametric analysis. Am J Nucl Med Mol Imaging. 2018;8:351–9.
Wu J, Liu H, Ye Q, Gallezot JD, Naganawa M, Miao T, et al. Generation of parametric Ki images for FDG PET using two 5-min scans. Med Phys. 2021;48:5219–31.
Khamwan K, Sukprakun C, Limotai C, Jirasakuldej S, Jantarato A, Hemachudha T, et al. Dynamic 18F-FDG-PET kinetic parameters for epileptogenic zone localization in drug-resistant epilepsy. Front Phys. 2023;11:1233059.
Tang Y, Liow J-S, Zhang Z, Li J, Long T, Li Y, et al. The evaluation of dynamic FDG-PET for detecting epileptic foci and analyzing reduced glucose phosphorylation in refractory epilepsy. Front Neurosci. 2019;12:993.
Croteau E, Lavallée E, Labbe SM, Hubert L, Pifferi F, Rousseau JA, et al. Image-derived input function in dynamic human PET/CT: methodology and validation with 11C-acetate and 18F-fluorothioheptadecanoic acid in muscle and 18F-fluorodeoxyglucose in brain. Eur J Nucl Med Mol Imaging. 2010;37:1539–50.
Volpi T, Maccioni L, Colpo M, Debiasi G, Capotosti A, Ciceri T, et al. An update on the use of image-derived input functions for human PET studies: new hopes or old illusions? EJNMMI Res. 2023;13:97.
Christiaan S, Carl KH, Johan N, Marc S, Christine W, Sung-Cheng H, et al. 1–11C-acetate kinetics of prostate cancer. J Nucl Med. 2008;49:206–15.
Driscoll B, Shek T, Vines D, Sun A, Jaffray D, Yeung I. Phantom validation of a conservation of activity-based partial volume correction method for arterial input function in dynamic PET imaging. Tomography. 2022;8:842–57.
Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39:904–11.
Rousset OG, Collins DL, Rahmim A, Wong DF. Design and implementation of an automated partial volume correction in PET: application to dopamine receptor quantification in the normal human striatum. J Nucl Med. 2008;49:1097–106.
Hong YT, Fryer TD. Kinetic modelling using basis functions derived from two-tissue compartmental models with a plasma input function: general principle and application to [18F]fluorodeoxyglucose positron emission tomography. Neuroimage. 2010;51:164–72.
Sokoloff L, Reivich M, Kennedy C, Rosiers MHD, Patlak CS, Pettigrew KD, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916.
Chen R, Ng YL, Zhao H, Ge Q, Shi F, Cao K, et al. Quantitative parametric imaging added diagnostic values in lesion detection of 68Ga-PSMA-11 in prostate cancer patients identified by dynamic total-body PET/CT. J Nucl Med. 2022;63(supplement 2):2584.
Sachpekidis C, Kopka K, Eder M, Hadaschik BA, Freitag MT, Pan L, et al. 68Ga-PSMA-11 dynamic PET/CT imaging in primary prostate cancer. Clin Nucl Med. 2016;41:e473–9.
Strauss DS, Sachpekidis C, Kopka K, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A. Pharmacokinetic studies of [68 Ga]Ga-PSMA-11 in patients with biochemical recurrence of prostate cancer: detection, differences in temporal distribution and kinetic modelling by tissue type. Eur J Nucl Med Mol Imaging. 2021;48:4472–82.
Kidera D, Kihara K, Akamatsu G, Mikasa S, Taniguchi T, Tsutsui Y, et al. The edge artifact in the point-spread function-based PET reconstruction at different sphere-to-background ratios of radioactivity. Ann Nucl Med. 2016;30:97–103.
Ilan E, Sandström M, Velikyan I, Sundin A, Eriksson B, Lubberink M. Parametric net influx rate images of 68Ga-DOTATOC and 68Ga-DOTATATE: quantitative accuracy and improved image contrast. J Nucl Med. 2017;58:744–9.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. 68Ga-PSMA PET/CT Joint EANM and SNMMI procedure guideline for prostate cancer imaging version 1.0. Eur J Nucl Med Mol Imaging. 2017;44(6):1014–24.
Dimitrakopoulou-Strauss A, Strauss LG, Heichel T, Wu H, Burger C, Bernd L, et al. The role of quantitative 18F-FDG PET studies for the differentiation of malignant and benign bone lesions. J Nucl Med. 2002;43:510–8.
Gallezot J-D, Lu Y, Naganawa M, Carson RE. Parametric imaging with PET and SPECT. IEEE Trans Radiat Plasma Med Sci. 2020;4:1–23.
Zuo Y, Qi J, Wang G. Relative patlak plot for dynamic PET parametric imaging without the need for early-time input function. Phys Med Biol. 2018;63:165004.
Acknowledgements
The authors appreciate the invaluable guidance and support provided by the nuclear medicine physicians, medical physicists, radiological technologists, and the dedicated team at the Division of Nuclear Medicine, Department of Radiology, King Chulalongkorn Memorial Hospital. Further, the authors would like to thank Prof. Dr. Guobao Wang from UC Davis for his valuable suggestion is this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Burasothikul, P., Navikhacheevin, C., Pasawang, P. et al. Dual-time-point dynamic 68Ga-PSMA-11 PET/CT for parametric imaging generation in prostate cancer. Ann Nucl Med (2024). https://doi.org/10.1007/s12149-024-01939-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12149-024-01939-z