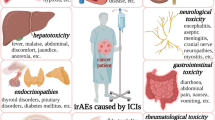
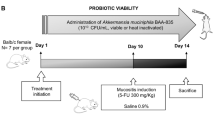
Abstract
Tumor is accompanied by complex and dynamic microenvironment development, and the interaction of all its components influences disease progression and response to treatment. Once the tumor microenvironment has been eradicated, various mechanisms can induce the tumors. Microorganisms can maintain the homeostasis of the tumor microenvironment through immune regulation, thereby inhibiting tumor development. Akkermania muciniphila (A. muciniphila), an anaerobic bacterium, can induce tumor immunity, regulate the gastrointestinal microenvironment through metabolites, outer membrane proteins, and some cytokines, and enhance the curative effect through combined immunization. Therefore, a comprehensive understanding of the complex interaction between A. muciniphila and human immunity will facilitate the development of immunotherapeutic strategies in the future and enable patients to obtain a more stable clinical response. This article reviews the most recent developments in the tumor immunity of A. muciniphila.



Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- A. muciniphila :
-
Akkermania muciniphila
- AmEVs:
-
A. muciniphila-Derived EVs
- CDDP:
-
Cisplatin
- CAR:
-
Chimeric antigen receptor
- CAR-T immunotherapy:
-
Chimeric antigen receptor T-cell immunotherapy
- CE:
-
Cranberry extract
- CRC:
-
Colorectal Cancer
- CTLs:
-
Cytotoxic T lymphocytes
- CXCR6:
-
CXC–chemokine receptor 6
- CXCL16:
-
CXC–chemokine ligand 16
- CTLA-4:
-
Cytotoxic T lymphocyte antigen 4
- DCs:
-
Dendritic cells
- FAS:
-
Factor-associated suicide
- FMT:
-
Fecal microbiota transplantation
- GPCR41:
-
G protein-coupled receptors 41
- GPCR43:
-
G protein-coupled receptors 43
- GPCR109A:
-
G protein-coupled receptors 109A
- GLP-1:
-
Glucagon-like peptide-1
- H3K14ac:
-
Lys14 on histone H3
- HSP70:
-
Heat shock protein 70
- HDAc:
-
Histone deacetylation
- HCC:
-
Hepatocellular carcinoma
- IgA:
-
Immunoglobulin A
- IgG1:
-
Immunoglobulin G1
- IL-1β:
-
Interleukin 1β
- IL-2:
-
Interleukin 2
- IL-6:
-
Interleukin 6
- IL-8:
-
Interleukin 8
- IL-10:
-
Interleukin 10
- IL-12:
-
Interleukin 12
- IL-18:
-
Interleukin 18
- IFN-γ:
-
Interferon gamma
- ICIs:
-
Immune checkpoint inhibitors
- LAG-3:
-
Lymphocyte activating gene 3
- M1-Like TAMs:
-
M1-like macrophages
- Muc3:
-
Mucin3
- Muc2:
-
Mucin2
- MDSCs:
-
Myeloid-derived suppressor cells
- MHC:
-
Major histocompatibility complex molecules
- NO:
-
Nitric oxide
- NLRP3:
-
Thermal protein domain associated protein 3
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Non-alcoholic hepatitis
- PYY:
-
Peptide YY
- p53:
-
Protein 53
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- PBMCs:
-
Peripheral blood mononuclear cells
- PD-1:
-
Programmed death receptor 1
- PD-L1:
-
Programmed death ligand 1
- SERT:
-
Serotonin reuptake transporter
- SCFAs:
-
Short-chain fatty acids
- TNF-α:
-
Tumor necrosis factor-α
- Treg cells:
-
Regulatory T cells
- TLR2:
-
Toll-like receptor 2
- Th1:
-
T helper 1
- TGF-β:
-
Transforming growth factor-β
- TME:
-
Tumor Microenvironment
- TFH cells:
-
T follicular helper cells
- TJPs:
-
Tight junction proteins
- TRAIL:
-
Tumor-necrosis-factor-related apoptosis-inducing ligand
- ZO-1:
-
Zonula Occludens-1
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Kuo CL, Chou HY, Chiu YC, Cheng AN, Fan CC, Chang YN, et al. Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. 2020;474:138–50.
Weng YS, Tseng HY, Chen YA, Shen PC, Al Haq AT, Chen LM, et al. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol Cancer. 2019;18(1):42.
Wang J, Sun J, Liu LN, Flies DB, Nie X, Toki M, et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med. 2019;25(4):656–66.
Wong TL, Ng KY, Tan KV, Chan LH, Zhou L, Che N, et al. CRAF methylation by prmt6 regulates aerobic glycolysis-driven hepatocarcinogenesis via erk-dependent pkm2 nuclear relocalization and activation. Hepatology. 2020;71(4):1279–96.
Boulch M, Grandjean CL, Cazaux M, Bousso P. Tumor immunosurveillance and immunotherapies: a fresh look from intravital imaging. Trends Immunol. 2019;40(11):1022–34.
Jiang X, Wang J, Deng X, **ong F, Ge J, **ang B, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18(1):10.
Tang S, Qin C, Hu H, Liu T, He Y, Guo H, et al. Immune checkpoint inhibitors in non-small cell lung cancer: progress, challenges, and prospects. Cells. 2022;11(3):320.
Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Jogalekar MP, Rajendran RL, Khan F, Dmello C, Gangadaran P, Ahn BC. CAR T-Cell-Based gene therapy for cancers: new perspectives, challenges, and clinical developments. Front Immunol. 2022;13: 925985.
Huuhtanen J, Kasanen H, Peltola K, Lönnberg T, Glumoff V, Brück O, et al. Single-cell characterization of anti-LAG-3 and anti-PD-1 combination treatment in patients with melanoma. J Clin Invest. 2023. https://doi.org/10.1172/JCI164809.
Zettl M, Wurm M, Schaaf O, Mostböck S, Tirapu I, Apfler I, et al. Combination of two novel blocking antibodies, anti-PD-1 antibody ezabenlimab (BI 754091) and anti-LAG-3 antibody BI 754111, leads to increased immune cell responses. Oncoimmunology. 2022;11(1):2080328.
Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7.
Becken B, Davey L, Middleton DR, Mueller KD, Sharma A, Holmes ZC et al: Genotypic and Phenotypic Diversity among Human Isolates of Akkermansia muciniphila. mBio 2021, doI: https://doi.org/10.1128/mBio.00478-21
Kobyliak N, Falalyeyeva T, Kyriachenko Y, Tseyslyer Y, Kovalchuk O, Hadiliia O, et al. Akkermansia muciniphila as a novel powerful bacterial player in the treatment of metabolic disorders. Minerva Endocrinol (Torino). 2022;47(2):242–52.
Kim JS, Kang SW, Lee JH, Park SH, Lee JS. The evolution and competitive strategies of akkermansia muciniphila in gut. Gut Microbes. 2022;14(1):2025017.
Zhang T, Li Q, Cheng L, Buch H, Zhang F. Akkermansia muciniphila is a promising probiotic. Microb Biotechnol. 2019;12(6):1109–25.
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–13.
Zhao Q, Yu J, Hao Y, Zhou H, Hu Y, Zhang C, et al. akkermansia muciniphila plays critical roles in host health. Crit Rev Microbiol. 2022;49(1):82–100.
Pietrzak B, Tomela K, Olejnik-Schmidt A, Mackiewicz A, Schmidt M. Secretory IgA in intestinal mucosal secretions as an adaptive barrier against microbial cells. Int J Mol Sci. 2020;21(23):9254.
Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 2019;364(6446):1179–84.
Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond). 2015;39(3):424–9.
Cani PD, Knauf C. A newly identified protein from akkermansia muciniphila stimulates GLP-1 secretion. Cell Metab. 2021;33(6):1073–5.
Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57(12):3126–35.
Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, et al. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004;287(6):G1168-1174.
de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–32.
Zhang WH, Jiang Y, Zhu QF, Gao F, Dai SF, Chen J, et al. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br Poult Sci. 2011;52(3):292–301.
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5.
Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734.
Ottman N, Reunanen J, Meijerink M, Pietilä TE, Kainulainen V, Klievink J, et al. Pili-like proteins of akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE. 2017;12(3): e0173004.
Wang J, Xu W, Wang R, Cheng R, Tang Z, Zhang M. The outer membrane protein Amuc_1100 of akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021;12(8):3597–610.
Zheng X, Huang W, Li Q, Chen Y, Wu L, Dong Y, et al. Membrane protein amuc_1100 derived from akkermansia muciniphila facilitates lipolysis and browning via activating the ac3/pka/hsl pathway. Microbiol Spectr. 2023;11(2): e0432322.
König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7(10): e196.
Shin J, Noh JR, Chang DH, Kim YH, Kim MH, Lee ES, et al. Elucidation of akkermansia muciniphila probiotic traits driven by mucin depletion. Front Microbiol. 2019;10:1137.
Chen T, Wang R, Duan Z, Yuan X, Ding Y, Feng Z, et al. Akkermansia muciniphila protects against psychological disorder-induced gut microbiota-mediated colonic mucosal barrier damage and aggravation of colitis. Front Cell Infect Microbiol. 2021;11: 723856.
Ahmadi Badi S, Moshiri A, Fateh A, Rahimi Jamnani F, Sarshar M, Vaziri F, et al. Microbiota-derived extracellular vesicles as new systemic regulators. Front Microbiol. 2017;8:1610.
Fábrega MJ, Aguilera L, Giménez R, Varela E, Alexandra Cañas M, Antolín M, et al. Activation of immune and defense responses in the intestinal mucosa by outer membrane vesicles of commensal and probiotic escherichia coli strains. Front Microbiol. 2016;7:705.
Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50(2): e450.
Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, et al. Extracellular vesicles derived from gut microbiota, especially akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE. 2013;8(10): e76520.
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–71.
Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al. Supplementation with akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–103.
Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, et al. Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2005;54(12):3358–70.
**itore A, Chambers ES, Hill T, Maldonado IR, Liu B, Bewick G, et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes Metab. 2017;19(2):257–65.
Heintz-Buschart A, Pandey U, Wicke T, Sixel-Döring F, Janzen A, Sittig-Wiegand E, et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33(1):88–98.
Hou Y, Li X, Liu C, Zhang M, Zhang X, Ge S, et al. Neuroprotective effects of short-chain fatty acids in MPTP induced mice model of Parkinson’s disease. Exp Gerontol. 2021;150: 111376.
Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728-1741.e1713.
Bárcena C, Valdés-Mas R, Mayoral P, Garabaya C, Durand S, Rodríguez F, et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med. 2019;25(8):1234–42.
Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572(7770):474–80.
Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81(11):3655–62.
Lungulescu CV, Răileanu S, Afrem G, Ungureanu BS, Florescu DN, Gheonea IA, et al. Histochemical and immunohistochemical study of mucinous rectal carcinoma. J Med Life. 2017;10(2):139–43.
Mall AS, Chirwa N, Govender D, Lotz Z, Tyler M, Rodrigues J, et al. MUC2, MUC5AC and MUC5B in the mucus of a patient with pseudomyxoma peritonei: biochemical and immunohistochemical study. Pathol Int. 2007;57(8):537–47.
Meng X, Wang W, Lan T, Yang W, Yu D, Fang X, et al. A purified aspartic protease from akkermansia muciniphila plays an important role in degrading muc2. Int J Mol Sci. 2019;21(1):72.
Gupta S. Molecular signaling in death receptor and mitochondrial pathways of apoptosis (review). Int J Oncol. 2003;22(1):15–20.
Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7(12):a006080.
Jiang Y, Xu Y, Zheng C, Ye L, Jiang P, Malik S, et al. Acetyltransferase from akkermansia muciniphila blunts colorectal tumourigenesis by reprogramming tumour microenvironment. Gut. 2023;72(7):1308–18.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85.
Borrelli A, Bonelli P, Tuccillo FM, Goldfine ID, Evans JL, Buonaguro FM, et al. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox Biol. 2018;15:467–79.
Li T, Lin X, Shen B, Zhang W, Liu Y, Liu H, et al. Akkermansia muciniphila suppressing nonalcoholic steatohepatitis associated tumorigenesis through CXCR6(+) natural killer T cells. Front Immunol. 2022;13:1047570.
Qu S, Fan L, Qi Y, Xu C, Hu Y, Chen S, et al. Akkermansia muciniphila alleviates dextran sulfate sodium (dss)-induced acute colitis by nlrp3 activation. Microbiol Spectr. 2021;9(2): e0073021.
Fan L, Xu C, Ge Q, Lin Y, Wong CC, Qi Y, et al. A muciniphila suppresses colorectal tumorigenesis by inducing tlr2/nlrp3-mediated m1-like tams. Cancer Immunol Res. 2021;9(10):1111–24.
Yao X, Zhang C, **ng Y, Xue G, Zhang Q, Pan F, et al. Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat Commun. 2017;8(1):1896.
Pitt JM, Vétizou M, Daillère R, Roberti MP, Yamazaki T, Routy B, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44(6):1255–69.
Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to pd-1 inhibition. N Engl J Med. 2017;377(25):2500–1.
Zitvogel L, Galluzzi L, Viaud S, Vétizou M, Daillère R, Merad M, et al. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7(271):271271.
Berman D, Parker SM, Siegel J, Chasalow SD, Weber J, Galbraith S, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11.
Yang H, Yao Z, Zhou X, Zhang W, Zhang X, Zhang F. Immune-related adverse events of checkpoint inhibitors: insights into immunological dysregulation. Clin Immunol. 2020;213: 108377.
Liang X, Ye X, Wang C, **ng C, Miao Q, **e Z, et al. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J Control Release. 2019;296:150–61.
Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 2023;79(2):506–15.
Bentebibel SE, Hurwitz ME, Bernatchez C, Haymaker C, Hudgens CW, Kluger HM, et al. A first-in-human study and biomarker analysis of nktr-214, a novel il2rβγ-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 2019;9(6):711–21.
Grenier JM, Yeung ST, Khanna KM. Combination immunotherapy: taking cancer vaccines to the next level. Front Immunol. 2018;9:610.
Bonmassar E, Testorelli C, Franco P, Goldin A, Cudkowicz G. Changes of the immunogenic properties of a radiation-induced mouse lymphoma following treatment with antitumor drugs. Cancer Res. 1975;35(8):1957–62.
Park SD, Kim CH, Kim CK, Park JA, Sohn HJ, Hong YK, et al. Cross-priming by temozolomide enhances antitumor immunity of dendritic cell vaccination in murine brain tumor model. Vaccine. 2007;25(17):3485–91.
Berd D. Low doses of chemotherapy to inhibit suppressor T cells. Prog Clin Biol Res. 1989;288:449–58.
Pietras RJ, Fendly BM, Chazin VR, Pegram MD, Howell SB, Slamon DJ. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene. 1994;9(7):1829–38.
Hancock MC, Langton BC, Chan T, Toy P, Monahan JJ, Mischak RP, et al. A monoclonal antibody against the c-erbB-2 protein enhances the cytotoxicity of cis-diamminedichloroplatinum against human breast and ovarian tumor cell lines. Cancer Res. 1991;51(17):4575–80.
Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, et al. Intestinal akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022;28(2):315–24.
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9.
Lu X. Impact of IL-12 in Cancer. Curr Cancer Drug Targets. 2017;17(8):682–97.
Chen C, Lim D, Cai Z, Zhang F, Liu G, Dong C, et al. HDAC inhibitor HPTA initiates anti-tumor response by CXCL9/10-recruited CXCR3(+)CD4(+)T cells against PAHs carcinogenicity. Food Chem Toxicol. 2023;176: 113783.
Chen ZF, Xu Q, Ding JB, Zhang Y, Du R, Ding Y. CD4+CD25+Foxp3+ Treg and TGF-beta play important roles in pathogenesis of Uygur cervical carcinoma. Eur J Gynaecol Oncol. 2012;33(5):502–7.
Chen Z, Qian X, Chen S, Fu X, Ma G, Zhang A. Akkermansia muciniphila enhances the antitumor effect of cisplatin in lewis lung cancer mice. J Immunol Res. 2020;2020:2969287.
Shi L, Sheng J, Chen G, Zhu P, Shi C, Li B, et al. Combining IL-2-based immunotherapy with commensal probiotics produces enhanced antitumor immune response and tumor clearance. J Immunother Cancer. 2020;8(2):e000973.
Stein-Thoeringer CK, Saini NY, Zamir E, Blumenberg V, Schubert ML, Mor U, et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat Med. 2023;29(4):906–16.
Juárez-Fernández M, Porras D, Petrov P, Román-Sagüillo S, García-Mediavilla MV, Soluyanova P, et al. The synbiotic combination of akkermansia muciniphila and quercetin ameliorates early obesity and nafld through gut microbiota resha** and bile acid metabolism modulation. Antioxidants (Basel). 2021;10(12):2001.
Newsome RC, Gharaibeh RZ, Pierce CM, da Silva WV, Paul S, Hogue SR, et al. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 2022;14(1):35.
Zhang Z, Shi X, Ji J, Guo Y, Peng Q, Hao L, et al. Dihydroartemisinin increased the abundance of akkermansia muciniphila by YAP1 depression that sensitizes hepatocellular carcinoma to anti-PD-1 immunotherapy. Front Med. 2023;17(4):729–46.
Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased akkermansia spp population in the gut microbiota of mice. Gut. 2015;64(6):872–83.
Anhê FF, Nachbar RT, Varin TV, Vilela V, Dudonné S, Pilon G, et al. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol Metab. 2017;6(12):1563–73.
Alard J, Lehrter V, Rhimi M, Mangin I, Peucelle V, Abraham AL, et al. Beneficial metabolic effects of selected probiotics on diet-induced obesity and insulin resistance in mice are associated with improvement of dysbiotic gut microbiota. Environ Microbiol. 2016;18(5):1484–97.
Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, et al. An increase in the akkermansia spp population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–35.
Acknowledgements
The figures were created by BioRender (Biorender.Com).
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Funding
This work was supported by grants from the Graduate Research-Innovation Project in Jiangsu province (SJCX22_1816), the Graduate Research and Practice Innovation Plan of Graduate Education Innovation Project in Jiangsu Province (No. SJCX211644), Social development project of key R & D plan of Jiangsu Provincial Department of science and technology (BE2022773), and Hospital level management project of Subei People's Hospital YYGL202228, the Social Development-Health Care Project of Yangzhou, Jiangsu Province (No. YZ2021075).
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Tang, D. Akkermania muciniphila: a rising star in tumor immunology. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03493-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03493-6