Abstract
Background
Although the immune checkpoint inhibitors (ICIs) became a vital part of cancer care, many patients do not respond to treatment, indicating need for biomarkers. The Pan-Immune-Inflammation Value (PIV) is a recently developed peripheral blood count-based biomarker. Herein, we evaluated a PIV-based candidate scoring system as a prognostic biomarker in ICI-treated patients.
Methods
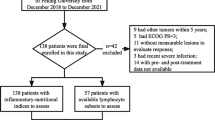
A total of 120 advanced cancer patients treated with anti-PD-1 or anti-PD-L1 inhibitors for any cancer type were included in this study. The PILE scoring system incorporating the PIV (< median vs. ≥ median), lactate dehydrogenase levels (normal vs. > normal) and Eastern Cooperative Oncology Group performance status (0 vs. ≥ 1) was constructed from the multivariate analyses and used for stratification. The association between overall survival (OS), progression-free survival and PILE risk category was evaluated with multivariate analysis.
Results
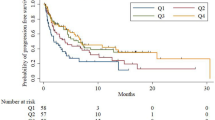
The median follow-up was 9.62 months and the median OS of all cohort were 12.42 ± 2.75 months. Patients with higher PIV had significantly decreased OS (7.75 ± 1.64 vs. 18.63 ± 4.26 months, p = 0.037). The patients in the PILE high-risk group (PILE score 2–3) had decreased OS (18.63 ± 4.02 vs. 5.09 ± 1.23 months, HR: 2.317, 95% CI: 1.450–3.700, p < 0.001) and PFS (7.69 ± 1.30 vs. 2.69 ± 0.65 months, HR: 1.931, 95% CI: 1.263–2.954, p = 0.002) compared to PILE low-risk group (PILE score 0–1). The Harrell C-Index values were 0.65 and 0.61 for OS and PFS prediction, respectively.
Conclusion
In this study, we demonstrated a decreased overall survival in ICI-treated patients with a higher PILE score. If prospective studies validate our results, PILE score could be a biomarker for immunotherapy.



Similar content being viewed by others
References
Kruger S, Ilmer M, Kobold S, Cadilha BL, Endres S, et al. Advances in cancer immunotherapy 2019–latest trends. J Exp Clin Cancer Res. 2019;38:268. https://doi.org/10.1186/s13046-019-1266-0.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. New Engl J Med. 2015;373:1803–13. https://doi.org/10.1056/NEJMoa1510665.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. New Engl J Med. 2019;381:1535–46. https://doi.org/10.1056/NEJMoa1910836.
Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–30. https://doi.org/10.1016/S0140-6736(18)32409-7.
Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. New Engl J Med. 2016;375:1856–67. https://doi.org/10.1056/NEJMoa1602252.
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, et al. PD-1 blockade with nivolumab in relapsed or refractory hodgkin’s lymphoma. New Engl J Med. 2014;372:311–9. https://doi.org/10.1056/NEJMoa1411087.
Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1547–57. https://doi.org/10.1016/S0140-6736(20)30230-0.
Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50:1–11. https://doi.org/10.1038/s12276-018-0191-1.
Ribas A. Releasing the brakes on cancer immunotherapy. New Engl J Med. 2015;373:1490–2. https://doi.org/10.1056/NEJMp1510079.
Gnjatic S, Bronte V, Brunet LR, Butler MO, Disis ML, et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. J ImmunoTherapy Cancer. 2017;5:44. https://doi.org/10.1186/s40425-017-0243-4.
Guven DC, Sahin TK, Dizdar O, Kilickap S (2020) Predictive biomarkers for immunotherapy efficacy in non-small-cell lung cancer: current status and future perspectives. Biomarkers Med 14:1383–1392. https://doi.org/10.2217/bmm-2020-0310
Hofman P, Heeke S, Alix-Panabières C, Pantel K. Liquid biopsy in the era of immuno-oncology: is it ready for prime-time use for cancer patients? Ann Oncol. 2019;30:1448–59. https://doi.org/10.1093/annonc/mdz196.
Nixon AB, Schalper KA, Jacobs I, Potluri S, Wang IM, et al. Peripheral immune-based biomarkers in cancer immunotherapy: can we realize their predictive potential? J Immunotherapy Cancer. 2019;7:325. https://doi.org/10.1186/s40425-019-0799-2.
Weide B, Martens A, Hassel JC, Berking C, Postow MA, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016;22:5487–96. https://doi.org/10.1158/1078-0432.Ccr-16-0127.
Tanizaki J, Haratani K, Hayashi H, Chiba Y, Nakamura Y, et al. Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol. 2018;13:97–105. https://doi.org/10.1016/j.jtho.2017.10.030.
Fucà G, Guarini V, Antoniotti C, Morano F, Moretto R, et al. The pan-immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer. 2020;123:403–9. https://doi.org/10.1038/s41416-020-0894-7.
Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23:141–8. https://doi.org/10.1016/j.semcancer.2013.02.005.
Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, et al. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia. 2014;16:771–88. https://doi.org/10.1016/j.neo.2014.08.013.
Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–34. https://doi.org/10.1038/nrc3004.
Riesenberg BP, Ansa-Addo EA, Gutierrez J, Timmers CD, Liu B, et al. Cutting edge: targeting thrombocytes to rewire anticancer immunity in the tumor microenvironment and potentiate efficacy of PD-1 blockade. J Immunol. 2019;203:1105–10. https://doi.org/10.4049/jimmunol.1900594.
Galli F, Aguilera JV, Palermo B, Markovic SN, Nisticò P, et al. Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J Exp Clin Cancer Res. 2020;39:89. https://doi.org/10.1186/s13046-020-01586-y.
Jeong J, Suh Y, Jung K. Context drives diversification of monocytes and neutrophils in orchestrating the tumor microenvironment. Front Immunol. 2019;10:1817–1817. https://doi.org/10.3389/fimmu.2019.01817.
Viñal D, Gutierrez-Sainz L, Martinez D, Garcia-Cuesta JA, Pedregosa J, et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced cancer patients receiving immunotherapy. Clin Transl Oncol. 2020. https://doi.org/10.1007/s12094-020-02509-1.
Bilen MA, Martini DJ, Liu Y, Shabto JM, Brown JT, et al. Combined effect of sarcopenia and systemic inflammation on survival in patients with advanced stage cancer treated with immunotherapy. Oncologist. 2020;25:e528–35. https://doi.org/10.1634/theoncologist.2019-0751.
Bigot F, Castanon E, Baldini C, Hollebecque A, Carmona A, et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: the Gustave Roussy Immune Score (GRIm-Score). Eur J Cancer. 2017;84:212–8. https://doi.org/10.1016/j.ejca.2017.07.027.
Prelaj A, Ferrara R, Rebuzzi SE, Proto C, Signorelli D et al (2019) EPSILoN: a prognostic score for immunotherapy in advanced non-small-cell lung cancer: a validation cohort. Cancers (Basel) 11. https://doi.org/10.3390/cancers11121954
Sen S, Hess K, Hong DS, Naing A, Piha-Paul S, et al. Development of a prognostic scoring system for patients with advanced cancer enrolled in immune checkpoint inhibitor phase 1 clinical trials. Br J Cancer. 2018;118:763–9. https://doi.org/10.1038/bjc.2017.480.
Maymani H, Hess K, Groisberg R, Hong DS, Naing A, et al. Predicting outcomes in patients with advanced non-small cell lung cancer enrolled in early phase immunotherapy trials. Lung Cancer. 2018;120:137–41. https://doi.org/10.1016/j.lungcan.2018.03.020.
Garrido-Laguna I, Janku F, Vaklavas C, Falchook GS, Fu S, et al. Validation of the Royal Marsden Hospital prognostic score in patients treated in the Phase I Clinical Trials Program at the MD Anderson Cancer Center. Cancer. 2012;118:1422–8. https://doi.org/10.1002/cncr.26413.
Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–7. https://doi.org/10.1001/jamaoncol.2017.4771.
Song P, Yang D, Cui X, Wang H, Si X et al (2020) NLCIPS: non-small cell lung cancer immunotherapy prognosis score. Cancer Manage Res 12:5975–5985. https://doi.org/10.2147/CMAR.S257967
Park W, Kwon D, Saravia D, Desai A, Vargas F, et al. Develo** a predictive model for clinical outcomes of advanced non-small cell lung cancer patients treated with nivolumab. Clin Lung Cancer. 2018;19(280–288):e284. https://doi.org/10.1016/j.cllc.2017.12.007.
Funding
The authors received no financial support for this article.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee of Hacettepe University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical approval
Due to the retrospective nature of the study, informed consent is not required for the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guven, D.C., Yildirim, H.C., Bilgin, E. et al. PILE: a candidate prognostic score in cancer patients treated with immunotherapy. Clin Transl Oncol 23, 1630–1636 (2021). https://doi.org/10.1007/s12094-021-02560-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02560-6