Abstract
Purpose
The application of nanosecond pulsed electric fields (nsPEFs) could be an effective therapeutic strategy for peritoneal metastasis (PM) from colorectal cancer (CRC). The aim of this study was to evaluate in vitro the sensitivity of CT-26 CRC cells to nsPEFs in combination with chemotherapeutic agents, and to observe the subsequent in vivo histologic response.
Methods
In vitro cellular assays were performed to assess the effects of exposure to 1, 10, 100, 500 and 1000 10 ns pulses in a cuvette or bi-electrode system at 10 and 200 Hz. nsPEF treatment was applied alone or in combination with oxaliplatin and mitomycin. Cell death was detected by flow cytometry, and permeabilization and intracellular calcium levels by fluorescent confocal microscopy after treatment. A mouse model of PM was used to investigate the effects of in vivo exposure to pulses delivered using a bi-electrode system; morphological changes in mitochondria were assessed by electron microscopy. Fibrosis was measured by multiphoton microscopy, while the histological response (HR; hematoxylin–eosin–safran stain), proliferation (KI67, DAPI), and expression of immunological factors (CD3, CD4, CD8) were evaluated by classic histology.
Results
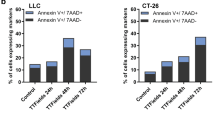
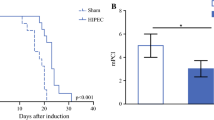
10 ns PEFs exerted a dose-dependent effect on CT-26 cells in vitro and in vivo, by inducing cell death and altering mitochondrial morphology after plasma membrane permeabilization. In vivo results indicated a specific CD8+ T cell immune response, together with a strong HR according to the Peritoneal Regression Grading Score (PRGS).
Conclusions
The effects of nsPEFs on CT-26 were confirmed in a mouse model of CRC with PM.







Similar content being viewed by others
Abbreviations
- nsPEFs:
-
Nanosecond pulsed electric fields
- PM:
-
Peritoneal metastasis
- CRC:
-
Colorectal cancer
- HES:
-
Hematoxylin and eosin stain
- HIPEC:
-
Hyperthermic intraperitoneal chemotherapy
- CT:
-
Control
- ns:
-
Non-significant
- PIPAC:
-
Pressurized Intraperitoneal Aerosol Chemotherapy
- PRGS:
-
Peritoneal Regression Grading Score
References
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JWW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. https://doi.org/10.1016/j.ejca.2012.12.027.
Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colon and rectum cancer National Cancer Institute. 2020 http://seer.cancer.gov/statfacts/html/colorect.html.
Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, Saltz L, Punt CJA, Koopman M, Tournigand C, Tebbutt NC, Diaz-Rubio E, Souglakos J, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17:1709–19. https://doi.org/10.1016/S1470-2045(16)30500-9.
Phelip JM, Tougeron D, Léonard D, Benhaim L, Desolneux G, Dupré A, Michel P, Penna C, Tournigand C, Louvet C, Christou N, Chevallier P, Dohan A, et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig Liver Dis. 2019;51:1357–63. https://doi.org/10.1016/j.dld.2019.05.035.
Pinto A, Pocard M. Hyperthermic intraperitoneal chemotherapy with cisplatin and mitomycin C for colorectal cancer peritoneal metastases: a systematic review of the literature. Pleura Peritoneum. 2019;4:20190006. https://doi.org/10.1515/pp-2019-0006.
Elias D, El Otmany A, Bonnay M, Paci A, Ducreux M, Antoun S, Lasser P, Laurent S, Bourget P. Human pharmacokinetic study of heated intraperitoneal oxaliplatin in increasingly hypotonic solutions after complete resection of peritoneal carcinomatosis. Oncology. 2002;63:346–52. https://doi.org/10.1159/000066229.
Elias D, Pocard M, Sideris L, Edè C, Ducreux M, Boige V, Lasser P. Preliminary results of intraperitoneal chemohyperthermia with oxaliplatin in peritoneal carcinomatosis of colorectal origin. Br J Surg. 2004;91:455–6. https://doi.org/10.1002/bjs.4399.
Elias D, Gilly F, Boutitie F, Quenet F, Bereder J-M, Mansvelt B, Lorimier G, Dubè P, Glehen O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–8. https://doi.org/10.1200/JCO.2009.23.9285.
Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S, Elias D, French Surgical Association. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608–18. https://doi.org/10.1002/cncr.25356.
Goéré D, Malka D, Tzanis D, Gava V, Boige V, Eveno C, Maggiori L, Dumont F, Ducreux M, Elias D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257:1065–71. https://doi.org/10.1097/SLA.0b013e31827e9289.
Miklavčič D, Mir LM, Thomas VP. Electroporation-based technologies and treatments. J Membrane Biol. 2010;236:1–2. https://doi.org/10.1007/s00232-010-9287-9.
Mir LM. Electroporation-based gene therapy: recent evolution in the mechanism description and technology developments. Methods Mol Biol. 2014;1121:3–23. https://doi.org/10.1007/978-1-4614-9632-8_1.
Davalos RV, Mir ILM, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223–31. https://doi.org/10.1007/s10439-005-8981-8.
Bardet SM, Carr L, Soueid M, Arnaud-Cormos D, Leveque P, O’Connor RP. Multiphoton imaging reveals that nanosecond pulsed electric fields collapse tumor and normal vascular perfusion in human glioblastoma xenografts. Sci Rep. 2016;6:34443. https://doi.org/10.1038/srep34443.
Miao X, Yin S, Shao Z, Zhang Y, Chen X. Nanosecond pulsed electric field inhibits proliferation and induces apoptosis in human osteosarcoma. J Orthop Surg Res. 2015;10:104. https://doi.org/10.1186/s13018-015-0247-z.
Cui G, Diao H. Research advances of anti-tumor immune response induced by pulse electric field ablation. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015;44:672–7.
Rossi A, Pakhomova ON, Mollica PA, Casciola M, Mangalanathan U, Pakhomov AG, Muratori C. Nanosecond pulsed electric fields induce endoplasmic reticulum stress accompanied by immunogenic cell death in murine models of lymphoma and colorectal cancer. Cancers. 2019. https://doi.org/10.3390/cancers11122034.
Chen X, James Swanson R, Kolb JF, Nuccitelli R, Schoenbach KH. Histopathology of normal skin and melanomas after nanosecond pulsed electric field treatment. Melanoma Res. 2009;19:361–71. https://doi.org/10.1097/CMR.0b013e32832f1558.
Chen X, Kolb JF, Swanson RJ, Schoenbach KH, Beebe SJ. Apoptosis initiation and angiogenesis inhibition: melanoma targets for nanosecond pulsed electric fields. Pigment Cell Melanoma Res. 2010;23:554–63. https://doi.org/10.1111/j.1755-148X.2010.00704.x.
Neumann E, Sowers AE, Jordan CA. Electroporation and electrofusion in cell biology. New York: Plenum Press; 1989.
Teissié A, Eynard A, Gabriel A, Rols A. Electropermeabilization of cell membranes. Adv Drug Deliv Rev. 1999;35:3–19. https://doi.org/10.1016/s0169-409x(98)00060-x.
Breton M, Mir LM. Microsecond and nanosecond electric pulses in cancer treatments. Bioelectromagnetics. 2012;33:106–23. https://doi.org/10.1002/bem.20692.
Pakhomov AG, Miklavcic D, Markov MS, editors. Advanced electroporation techniques in biology in medicine. Boca Raton: CRC Press; 2010.
Zimmermann U, Friedrich U, Mussauer H, Gessner P, Hämel K, Sukhorukov V. Electromanipulation of mammalian cells: fundamentals and application. IEEE Trans Plasma Sci. 2000;28(1):72–822000.
Tarek M. Membrane electroporation: a molecular dynamics simulation. Biophys J. 2005;88:4045–53. https://doi.org/10.1529/biophysj.104.050617.
Gowrishankar TR, Esser AT, Vasilkoski Z, Smith KC, Weaver JC. Microdosimetry for conventional and supra-electroporation in cells with organelles. Biochem Biophys Res Commun. 2006;341:1266–76. https://doi.org/10.1016/j.bbrc.2006.01.094.
Delemotte L, Tarek M. Molecular dynamics simulations of lipid membrane electroporation. J Membr Biol. 2012;245:531–43. https://doi.org/10.1007/s00232-012-9434-6.
Kotnik T, Rems L, Tarek M, Miklavčič D. Membrane electroporation and electropermeabilization: mechanisms and models. Annu Rev Biophys. 2019;48:63–91. https://doi.org/10.1146/annurev-biophys-052118-115451.
Cui C, Merritt R, Fu L, Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharm Sin B. 2017;7:3–17. https://doi.org/10.1016/j.apsb.2016.11.001.
Carr L, Bardet SM, Arnaud-Cormos D, Leveque P, O’Connor RP. Visualisation of an nsPEF induced calcium wave using the genetically encoded calcium indicator GCaMP in U87 human glioblastoma cells. Bioelectrochemistry. 2018;119:68–75. https://doi.org/10.1016/j.bioelechem.2017.09.003.
White JA, Blackmore PF, Schoenbach KH, Beebe SJ. Stimulation of capacitative calcium entry in HL-60 cells by nanosecond pulsed electric fields. J Biol Chem. 2004;279:22964–72. https://doi.org/10.1074/jbc.M311135200.
Vernier PT, Sun Y, Marcu L, Salemi S, Craft CM, Gundersen MA. Calcium bursts induced by nanosecond electric pulses. Biochem Biophys Res Commun. 2003;310:286–95. https://doi.org/10.1016/j.bbrc.2003.08.140.
Semenov I, **ao S, Pakhomov AG. Primary pathways of intracellular Ca(2+) mobilization by nanosecond pulsed electric field. Biochim Biophys Acta. 2013;1828:981–9. https://doi.org/10.1016/j.bbamem.2012.11.032.
Beebe SJ, Sain NM, Ren W. Induction of cell death mechanisms and apoptosis by nanosecond pulsed electric fields (nsPEFs). Cells. 2013;2:136–62. https://doi.org/10.3390/cells2010136.
Nuccitelli R, McDaniel A, Connolly R, Zelickson B, Hartman H. Nano-pulse stimulation induces changes in the intracellular organelles in rat liver tumors treated in situ. Lasers Surg Med. 2020;52(9):882–9. https://doi.org/10.1002/lsm.23239.
Ren Z, Chen X, Cui G, Yin S, Chen L, Jiang J, Hu Z, **e H, Zheng S, Zhou L. Nanosecond pulsed electric field inhibits cancer growth followed by alteration in expressions of NF-κB and Wnt/β-catenin signaling molecules. PLoS ONE. 2013;8:e74322. https://doi.org/10.1371/journal.pone.0074322.
Rossi A, Pakhomova ON, Pakhomov AG, Weygandt S, Bulysheva AA, Murray LE, Mollica PA, Muratori C. Mechanisms and immunogenicity of nsPEF-induced cell death in B16F10 melanoma tumors. Sci Rep. 2019;9:431. https://doi.org/10.1038/s41598-018-36527-5.
Xu X, Chen Y, Zhang R, Miao X, Chen X. Activation of anti-tumor Immune response by ablation of HCC with nanosecond pulsed electric field. J Clin Transl Hepatol. 2018;6:85–8. https://doi.org/10.14218/JCTH.2017.00042.
Chen R, Sain NM, Harlow KT, Chen Y-J, Shires PK, Heller R, Beebe SJ. A protective effect after clearance of orthotopic rat hepatocellular carcinoma by nanosecond pulsed electric fields. Eur J Cancer. 2014;50:2705–13. https://doi.org/10.1016/j.ejca.2014.07.006.
Nuccitelli R, Berridge JC, Mallon Z, Kreis M, Athos B, Nuccitelli P. Nanoelectroablation of murine tumors triggers a CD8-dependent inhibition of secondary tumor growth. PLoS ONE. 2015;10:e0134364. https://doi.org/10.1371/journal.pone.0134364.
Nuccitelli R, Tran K, Lui K, Huynh J, Athos B, Kreis M, Nuccitelli P, De Fabo EC. Non-thermal nanoelectroablation of UV-induced murine melanomas stimulates an immune response. Pigment Cell Melanoma Res. 2012;25:618–29. https://doi.org/10.1111/j.1755-148X.2012.01027.x.
Soueid M, Kohler S, Carr L, Bardet SM, O’Connor RP, Leveque P, Arnaud-Cormos P. Electromagnetic analysis of an aperture modified TEM cell including an ITO layer for real-time observation of biological cells exposed to microwaves. Progr Electromagn Res. 2014;149:193–204.
Kenaan M, El Amari S, Silve A, Merla C, Mir LM, Couderc V, Arnaud-Cormos D, Leveque P. Characterization of a 50- Ω exposure setup for high-voltage nanosecond pulsed electric field bioexperiments. IEEE Trans Biomed Eng. 2011;58:207–14. https://doi.org/10.1109/TBME.2010.2081670.
Wu Y-H, Arnaud-Cormos D, Casciola M, Sanders JM, Leveque P, Vernier PT. Moveable wire electrode microchamber for nanosecond pulsed electric-field delivery. IEEE Trans Biomed Eng. 2013;60:489–96. https://doi.org/10.1109/TBME.2012.2228650.
Taibi A, Albouys J, Jacques J, Perrin M-L, Yardin C, Durand Fontanier S, Bardet SM. Comparison of implantation sites for the development of peritoneal metastasis in a colorectal cancer mouse model using non-invasive bioluminescence imaging. PLoS ONE. 2019;14:e0220360. https://doi.org/10.1371/journal.pone.0220360.
Solass W, Sempoux C, Detlefsen S, Carr NJ, Bibeau F. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura and Peritoneum. 2016;1(2):99–107. https://doi.org/10.1515/pp-2016-0011.
Yin S, Chen X, Hu C, Zhang X, Hu Z, Yu J, Feng X, Jiang K, Ye S, Shen K, **e H, Zhou L, James Swanson R, et al. Nanosecond pulsed electric field (nsPEF) treatment for hepatocellular carcinoma: a novel locoregional ablation decreasing lung metastasis. Cancer Lett. 2014;346:285–91. https://doi.org/10.1016/j.canlet.2014.01.009.
Chen X, Yin S, Hu C, Chen X, Jiang K, Ye S, Feng X, Fan S, **e H, Zhou L, Zheng S. Comparative study of nanosecond electric fields in vitro and in vivo on hepatocellular carcinoma indicate macrophage infiltration contribute to tumor ablation in vivo. PLoS ONE. 2014;9:e86421. https://doi.org/10.1371/journal.pone.0086421.
Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, Kitamura H, Nishimura T. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 2010;70:2697–706. https://doi.org/10.1158/0008-5472.CAN-09-2982.
Abboud K, André T, Brunel M, Ducreux M, Eveno C, Glehen O, Goéré D, Gornet J-M, Lefevre JH, Mariani P, Pinto A, Quenet F, Sgarbura O, et al. Management of colorectal peritoneal metastases: expert opinion. J Visc Surg. 2019;156:377–9. https://doi.org/10.1016/j.jviscsurg.2019.08.002.
Alyami M, Hübner M, Grass F, Bakrin N, Villeneuve L, Laplace N, Passot G, Glehen O, Kepenekian V. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 2019;20:e368–77. https://doi.org/10.1016/S1470-2045(19)30318-3.
Hristov K, Mangalanathan U, Casciola M, Pakhomova ON, Pakhomov AG. Expression of voltage-gated calcium channels augments cell susceptibility to membrane disruption by nanosecond pulsed electric field. Biochim Biophys Acta Biomembr. 2018;1860:2175–83. https://doi.org/10.1016/j.bbamem.2018.08.017.
Nesin OM, Pakhomova ON, **ao S, Pakhomov AG. Manipulation of cell volume and membrane pore comparison following single cell permeabilization with 60- and 600-ns electric pulses. Biochim Biophys Acta. 2011;1808:792–801. https://doi.org/10.1016/j.bbamem.2010.12.012.
Pakhomov AG, Kolb JF, White JA, Joshi RP, **ao S, Schoenbach KH. Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF). Bioelectromagnetics. 2007;28:655–63. https://doi.org/10.1002/bem.20354.
Bowman AM, Nesin OM, Pakhomova ON, Pakhomov AG. Analysis of plasma membrane integrity by fluorescent detection of Tl(+) uptake. J Membr Biol. 2010;236:15–26. https://doi.org/10.1007/s00232-010-9269-y.
Creighton TE. Proteins: structures and molecular properties. New York: W.H. Freeman; 1993.
Nuccitelli R, Chen X, Pakhomov AG, Baldwin WH, Sheikh S, Pomicter JL, Ren W, Osgood C, Swanson RJ, Kolb JF, Beebe SJ, Schoenbach KH. A new pulsed electric field therapy for melanoma disrupts the tumor’s blood supply and causes complete remission without recurrence. Int J Cancer. 2009;125:438–45. https://doi.org/10.1002/ijc.24345.
Beebe SJ, White J, Blackmore PF, Deng Y, Somers K, Schoenbach KH. Diverse effects of nanosecond pulsed electric fields on cells and tissues. DNA Cell Biol. 2003;22:785–96. https://doi.org/10.1089/104454903322624993.
Beebe SJ, Fox PM, Rec LJ, Willis ELK, Schoenbach KH. Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J. 2003;17:1493–5. https://doi.org/10.1096/fj.02-0859fje.
Ford WE, Ren W, Blackmore PF, Schoenbach KH, Beebe SJ. Nanosecond pulsed electric fields stimulate apoptosis without release of pro-apoptotic factors from mitochondria in B16f10 melanoma. Arch Biochem Biophys. 2010;497:82–9. https://doi.org/10.1016/j.abb.2010.03.008.
Hall EH, Schoenbach KH, Beebe SJ. Nanosecond pulsed electric fields induce apoptosis in p53-wildtype and p53-null HCT116 colon carcinoma cells. Apoptosis. 2007;12:1721–31. https://doi.org/10.1007/s10495-007-0083-7.
Vincelette RL, Roth CC, McConnell MP, Payne JA, Beier HT, Ibey BL. Thresholds for phosphatidylserine externalization in Chinese hamster ovarian cells following exposure to nanosecond pulsed electrical fields (nsPEF). PLoS ONE. 2013;8:e63122. https://doi.org/10.1371/journal.pone.0063122.
Muratori C, Pakhomov AG, Gianulis E, Meads J, Casciola M, Mollica PA, Pakhomova ON. Activation of the phospholipid scramblase TMEM16F by nanosecond pulsed electric fields (nsPEF) facilitates its diverse cytophysiological effects. J Biol Chem. 2017;292:19381–91. https://doi.org/10.1074/jbc.M117.803049.
Pakhomova ON, Gregory BW, Semenov I, Pakhomov AG. Two modes of cell death caused by exposure to nanosecond pulsed electric field. PLoS ONE. 2013;8:e70278. https://doi.org/10.1371/journal.pone.0070278.
Mir LM, Orlowski S, Belehradek J, Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer. 1991;27:68–72.
García-Sánchez T, Leray I, Ronchetti M, Cadossi R, Mir LM. Impact of the number of electric pulses on cell electrochemotherapy in vitro: limits of linearity and saturation. Bioelectrochemistry. 2019;129:218–27. https://doi.org/10.1016/j.bioelechem.2019.05.021.
Wu S, Guo J, Wei W, Zhang J, Fang J, Beebe SJ. Enhanced breast cancer therapy with nsPEFs and low concentrations of gemcitabine. Cancer Cell Int. 2014;14:98. https://doi.org/10.1186/s12935-014-0098-4.
Morotomi-Yano K, Akiyama H, Yano K. Nanosecond pulsed electric fields activate MAPK pathways in human cells. Arch Biochem Biophys. 2011;515:99–106. https://doi.org/10.1016/j.abb.2011.09.002.
Morotomi-Yano K, Oyadomari S, Akiyama H, Yano K. Nanosecond pulsed electric fields act as a novel cellular stress that induces translational suppression accompanied by eIF2α phosphorylation and 4E-BP1 dephosphorylation. Exp Cell Res. 2012;318:1733–44. https://doi.org/10.1016/j.yexcr.2012.04.016.
Morotomi-Yano K, Akiyama H, Yano K. Nanosecond pulsed electric fields activate AMP-activated protein kinase: implications for calcium-mediated activation of cellular signaling. Biochem Biophys Res Commun. 2012;428:371–5. https://doi.org/10.1016/j.bbrc.2012.10.061.
Gao C, Zhang X, Chen J, Zhao J, Liu Y, Zhang J, Wang J. Utilizing the nanosecond pulse technique to improve antigen intracellular delivery and presentation to treat tongue squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2018;23:e344–50. https://doi.org/10.4317/medoral.22227.
Kaufman D, Martinez M, Jauregui L, Ebbers E, Nuccitelli R, Knape WA, Uecker D, Mehregan D. A dose-response study of a novel method of selective tissue modification of cellular structures in the skin with nanosecond pulsed electric fields. Lasers Surg Med. 2020;52:315–22. https://doi.org/10.1002/lsm.23145.
Nuccitelli R, Wood R, Kreis M, Athos B, Huynh J, Lui K, Nuccitelli P, Epstein EH. First-in-human trial of nanoelectroablation therapy for basal cell carcinoma: proof of method. Exp Dermatol. 2014;23:135–7. https://doi.org/10.1111/exd.12303.
Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. https://doi.org/10.1038/nri957.
Tougeron D, Fauquembergue E, Latouche J-B. Immune response and colorectal cancer. Bull Cancer. 2013;100:283–94. https://doi.org/10.1684/bdc.2013.1716.
Atreya I, Neurath MF. Immune cells in colorectal cancer: prognostic relevance and therapeutic strategies. Exp Rev Anticancer Ther. 2008;8:561–72. https://doi.org/10.1586/14737140.8.4.561.
Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE, Soubrane O, Chaussade S, Mentha G, Terris B. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. https://doi.org/10.1093/annonc/mdl386.
Passot G, You B, Boschetti G, Fontaine J, Isaac S, Decullier E, Maurice C, Vaudoyer D, Gilly F-N, Cotte E, Glehen O. Pathological response to neoadjuvant chemotherapy: a new prognosis tool for the curative management of peritoneal colorectal carcinomatosis. Ann Surg Oncol. 2014;21:2608–14. https://doi.org/10.1245/s10434-014-3647-0.
Bardet SM, Cortese J, Blanc R, Mounayer C, Rouchaud A. Multiphoton microscopy for pre-clinical evaluation of flow-diverter stents for treating aneurysms. J Neuroradiol. 2020. https://doi.org/10.1016/j.neurad.2020.03.005.
Taibi A, Lo Dico R, Kaci R, Naneix AL, Malgras B, Mathonnet M, Pocard M. Evaluation of a new histological grading system for assessing the response to chemotherapy of peritoneal metastases from colorectal cancer: a mouse model study. Eur J Surg Oncol. 2020;46:160–5. https://doi.org/10.1016/j.ejso.2019.09.008.
Benzerdjeb N, Durieux E, Tantot J, Isaac S, Fontaine J, Harou O, Glehen O, Kepenekian V, Alyami M, Villeneuve L, Laplace N, Traverse-Glehen A, Shisheboran-Devouassoux M, et al. Prognostic impact of combined progression index based on peritoneal grading regression score and peritoneal cytology in peritoneal metastasis. Histopathology. 2020;77(4):548–59. https://doi.org/10.1111/his.14092.
Taibi A, Dico R, Kaci R, Naneix AL, Mathonnet M, Pocard M. Impact of preoperative chemotherapy on the histological response of patients with peritoneal metastases from colorectal cancer according to peritoneal regression grading score (PRGS) and TRG. Surg Oncol. 2020;33:158–63. https://doi.org/10.1016/j.suronc.2020.02.014.
Lee ZJ, Chia SL, Tan G, Soo KC, Teo CCM. Cost effectiveness of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for management of colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2018;25:2340–6. https://doi.org/10.1245/s10434-018-6508-4.
Acknowledgements
We thank R Bachelot for his interest during his master for multiphoton microscopy/HIPEC, Pr. M Pocard (CART Inserm U965) for the technical support regarding cell culture and P Leveque and D Arnaud-Cormos for lending the exposure system. This work benefited from government support managed by the National Research Agency under the Investments for the future program with the reference ANR-10-LABX-0074-01 Sigma-LIM, and funds from the Limoges Hospital committee for research orientation in oncology (CORC « Carcinopulse » 2018). This research was conducted in the scope of GDR HappyBio (CNRS) and LEA-EBAM, a European Associated Laboratory titled “Pulsed Electric Fields Applications in Biology and Medicine”.
Funding
This work benefited from government support managed by the National Research Agency under the Investments for the future program with the reference ANR-10-LABX-0074–01 Sigma-LIM, and was also supported by funds from the Limoges Hospital committee for research orientation in oncology (CORC « Carcinopulse» 2018).
Author information
Authors and Affiliations
Contributions
Study conception and design: AT, SDF, SB. Provision of study materials or patients: AT, M-LP, SB. Data and statistical analysis and interpretation: AT and SB. Drafting and editing of manuscript: all authors. Critical manuscript review and approval of final version: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Research involving human participants and/or animals
The use of animals was approved by the local Ethics and Animal Care Committee (registration number: 2017102611003706, Sylvia M Bardet). All animal care and experimental procedures were conducted in conformity with 2013 French legislation, which is in accordance with European Community guidelines (directive 2010/63/UE for the Care and Use of Laboratory Animals).
Informed consent
For this study, we obtained the consent for all the patients
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12094_2020_2525_MOESM1_ESM.tiff
Supplementary file1 Supplementary Figure 1: Dose-dependant results for mitomycin and oxaliplatin. Cell viability was assessed by MTT assays after 24 h (white dots) and 48 h (black dots) of treatment (data for 72 h are not shown). The IC50 values for the 24-h treatment were 17.5 µg/ml for mitomycin and 200 µg/ml for oxaliplatin. (TIFF 2275 KB)
Rights and permissions
About this article
Cite this article
Taibi, A., Perrin, ML., Albouys, J. et al. 10 ns PEFs induce a histological response linked to cell death and cytotoxic T-lymphocytes in an immunocompetent mouse model of peritoneal metastasis. Clin Transl Oncol 23, 1220–1237 (2021). https://doi.org/10.1007/s12094-020-02525-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02525-1