Abstract
Background
Bisphosphonates are the mainstay of osteoporosis treatment, but their use for patients with esophageal varices has been avoided due to the risk of esophagitis, which may cause variceal bleeding. Since most clinical trials assessing osteoporosis treatment last 2–3 years, this study aimed to evaluate a 2-year risedronate treatment for patients with esophageal varices and liver cirrhosis.
Methods
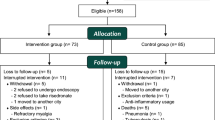
The study received Institutional Review Board approval, and the sample was divided into two groups according to bone mineral density (BMD). Cirrhosis severity and endoscopic findings at baseline were similar between the groups. The intervention group had 51 patients with osteoporosis, who received oral risedronate 35 mg weekly plus calcium and vitamin D supplements. The control group had 51 patients with osteopenia, receiving only the supplements. Scheduled esophagogastroduodenoscopies and BMD measurements were carried out.
Results
The adjusted esophagitis risk was higher in the intervention group; however, none of the subjects had digestive bleeding. Lumbar spine BMD increased in the intervention group (− 3.06 ± 0.71 to − 2.33 ± 0.90; p < 0.001) and in the control group (− 1.38 ± 0.77 to − 1.10 ± 1.05; p = 0.012). Femoral neck BMD did not change in the intervention group (− 1.64 ± 0.91 to − 1.71 ± 0.95; p = 0.220), but tended to decrease in the control group (− 1.00 ± 0.74 to − 1.09 ± 0.82; p = 0.053).
Conclusion
Oral risedronate was effective and did not cause gastrointestinal bleeding in cirrhotic patients with esophageal varices under endoscopic surveillance.
Graphic abstract



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMD:
-
Bone mineral density
- DXA:
-
Dual-energy X-ray absorptiometry
- EVBL:
-
Endoscopic variceal band ligation
- PHG:
-
Portal hypertensive gastropathy
- UGIB:
-
Upper gastrointestinal bleeding
References
Huldén E, Castedal M, Karlsson MK, Kalaitzakis E, Swärd P. Osteoporosis in cirrhotics before and after liver transplantation: relation with malnutrition and inflammatory status. Scand J Gastroenterol 2020;55(3):354–361. https://doi.org/10.1080/00365521.2020.1735507
Jeong HM, Kim DJ. Bone diseases in patients with chronic liver disease. Int J Mol Sci 2019;20(17):4270. https://doi.org/10.3390/ijms20174270
Santos LA, Romeiro FG. Diagnosis and management of cirrhosis-related osteoporosis. Biomed Res Int 2016;2016:1423462. https://doi.org/10.1155/2016/1423462
Kanis JA, Cooper C, Rizzoli R, Reginster JY. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 2019;30(1):3–44. https://doi.org/10.1007/s00198-018-4704-5
Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract 2020;26(Suppl 1):1–46. https://doi.org/10.4158/GL-2020-0524SUPPL
Bansal RK, Kumar M, Sachdeva PR, Kumar A. Prospective study of profile of hepatic osteodystrophy in patients with non-choleastatic liver cirrhosis and impact o. United European Gastroenterol J 2016;4(1):77–83. https://doi.org/10.1177/2050640615584535
Lima TB, Santos LAA, Nunes HRC, Silva GF, Caramori CA, Qi X, et al. Safety and efficacy of risedronate for patients with esophageal varices and liver cirrhosis: a non-randomized clinical trial. Sci Rep 2019;9(1):18958. https://doi.org/10.1038/s41598-019-55603-y
Senn C, Günther B, Popp AW, Perrelet R, Hans D, Lippuner K. Comparative effects of teriparatide and ibandronate on spine bone mineral density (BMD) and microarchitecture (TBS) in postmenopausal women with osteoporosis: a 2-year open-label study. Osteoporos Int 2014;25(7):1945–1951. https://doi.org/10.1007/s00198-014-2703-8
McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344(5):333–40. https://doi.org/10.1056/NEJM200102013440503
McClung MR, Zanchetta JR, Racewicz A, Roux C, Benhamou CL, Man Z, et al. Efficacy and safety of risedronate 150-mg once a month in the treatment of postmenopausal osteoporosis: 2-year data. Osteoporos Int 2013;24(1):293–9. https://doi.org/10.1007/s00198-012-2056-0
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000;11(1):83–91. https://doi.org/10.1007/s001980050010
Kanis JA. An update on the diagnosis of osteoporosis. Curr Rheumatol Rep 2000;2(1):62–66. https://doi.org/10.1007/s11926-996-0070-y
Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res 1994;9(8):1137–1141. https://doi.org/10.1002/jbmr.5650090802
Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, Kokudo N,et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc 2010;22(1):1–9. https://doi.org/10.1111/j.1443-1661.2009.00929.x
Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45(2):172–180. https://doi.org/10.1136/gut.45.2.172
Sakita T. Endoscopy in diagnosis of early gastric cancer. Clin Gastroenterol 1973;2:345–360
McCormack TT, Sims J, Eyre-Brook I, Kennedy H, Goepel J, Johnson AG, et al. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut 1985;26(11):1226–1232. https://doi.org/10.1136/gut.26.11.1226
Yamamoto K, Kishino M, Nakamura S, Tokushige K. Symptoms and upper gastrointestinal mucosal injury associated with bisphosphonate therapy. Intern Med 2019;58(8):1049–1056. https://doi.org/10.2169/internalmedicine.1271-18
Kang JH, Keller JJ, Lin HC. Bisphosphonates reduced the risk of acute myocardial infarction: a 2-year follow-up study. Osteoporos Int 2013;24(1):271–277. https://doi.org/10.1007/s00198-012-2213-5
Kang JH, Keller JJ, Lin HC. A population-based 2-year follow-up study on the relationship between bisphosphonates and the risk of stroke. Osteoporos Int 2012;23(10):2551–2557. https://doi.org/10.1007/s00198-012-1894-0
Peris P, Parés A, Guañabens N, Del Río L, Pons F, Martínez de Osaba MJ, Monegal A, et al. Bone mass improves in alcoholics after 2 years of abstinence. J Bone Miner Res 1994;9(10):1607–12. https://doi.org/10.1002/jbmr.5650091014.
Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int 2012;23(1):1–16. https://doi.org/10.1007/s00198-011-1787-7
Yip TC, Lee HW, Wong VW, Wong GL, Tse YK, Lui GC, et al. Factors associated with improvement in MELD score after antiviral treatment in patients with chronic hepatitis B. J Gastroenterol Hepatol 2020;35(9):1610–1618. https://doi.org/10.1111/jgh.15007
Pascasio JM, Vinaixa C, Ferrer MT, Colmenero J, Rubin A, Castells L, et al. Clinical outcomes of patients undergoing antiviral therapy while awaiting liver transplantation. J Hepatol 2017;67(6):1168–1176. https://doi.org/10.1016/j.jhep.2017.08.008
Belli LS, Berenguer M, Cortesi PA, Strazzabosco M, Rockenschaub SR, Martini S, Morelli C, et al.. Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J Hepatol 2016;65(3):524–31. https://doi.org/10.1016/j.jhep.2016.05.010
Aravinthan AD, Barbas AS, Doyle AC, Tazari M, Sapisochin G, Cattral MS, et al. Characteristics of liver transplant candidates delisted following recompensation and predictors of such delisting in alcohol-related liver disease: a case–control study. Transpl Int 2017;30(11):1140–1149. https://doi.org/10.1111/tri.13008
Zaman A, Hapke R, Flora K, Rosen H, Benner K. Prevalence of upper and lower gastrointestinal tract findings in liver transplant candidates undergoing screening endoscopic evaluation. Am J Gastroenterol 1999;94(4):895–899. https://doi.org/10.1111/j.1572-0241.1999.984_g.x
Zhang J, Cui PL, Lv D, Yao SW, Xu YQ, Yang ZX. Gastroesophageal reflux in cirrhotic patients without esophageal varices. World J Gastroenterol 2011;17(13):1753–1758. https://doi.org/10.3748/wjg.v17.i13.1753
Garbuzenko DV, Arefyev NO. Primary prevention of bleeding from esophageal varices in patients with liver cirrhosis: an update and review of the literature. J Evid Based Med 2020;13(4):313–324. https://doi.org/10.1111/jebm.12407
de Franchis R, Faculty BVI. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63(3):743–752. https://doi.org/10.1016/j.jhep.2015.05.022
Dultz G, Piiper A, Zeuzem S, Kronenberger B, Waidmann O. Proton pump inhibitor treatment is associated with the severity of liver disease and increased mortality in patients with cirrhosis. Aliment Pharmacol Ther 2015;41(5):459–466. https://doi.org/10.1111/apt.13061
Cole HL, Pennycook S, Hayes PC. The impact of proton pump inhibitor therapy on patients with liver disease. Aliment Pharmacol Ther 2016;44(11–12):1213–1223. https://doi.org/10.1111/apt.13827
Kishimoto H, Maehara M. Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: analysis of data from the CISA. Arch Osteoporos 2015;10:231. https://doi.org/10.1007/s11657-015-0231-6.
Mankal PK, Abed J, Aristy JD, Munot K, Suneja U, Engelson ES, et al. Relative effects of heavy alcohol use and hepatitis C in decompensated chronic liver disease in a hospital inpatient population. Am J Drug Alcohol Abuse 2015;41(2):177–182. https://doi.org/10.3109/00952990.2014.964358
Bassegoda O, Olivas P, Turco L, Mandorfer M, Serra-Burriel M, Tellez L, Kwanten W, et al.. Decompensation in advanced non-alcoholic fatty liver disease may occur at lower hepatic venous pressure gradient levels that in patients with viral disease. Clin Gastroenterol Hepatol 2021. https://doi.org/10.1016/j.cgh.2021.10.023
Acknowledgements
The authors would like to acknowledge the grant support received from the São Paulo Research Foundation (FAPESP) and from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Funding
This study was funded by São Paulo Research Foundation (FAPESP) through Grant Nos. 2016/07117-9 and 2014/22572-9.
Author information
Authors and Affiliations
Contributions
Lívia Alves Amaral Santos, Talles Bazeia Lima and Fernando Gomes Romeiro designed the study, conducted the patients, collected the data and drafted the manuscript. Hélio Rubens de Carvalho Nunes performed the statistical analysis. **ngshun Qi and Fernando Gomes Romeiro reviewed and modified the manuscript until achieving the final version of the article, which was approved by all the authors.
Corresponding author
Ethics declarations
Conflict of interest
Lívia Alves Amaral Santos, Talles Bazeia Lima, Hélio Rubens de Carvalho Nunes, **ngshun Qi and Fernando Gomes Romeiro declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (denoted “Comitê de Ética em Pesquisa” - protocol number 089211-2013) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Clinical trials registration
The project was registered in a publicly accessible primary International Clinical Trial Registry Platform (named REBEC clinical trials platform, available at http://www.ensaiosclinicos.gov.br), where the registration date and the trial registration number were 10/11/2015 and RBR-76pm35, respectively.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santos, L.A.A., Lima, T.B., de Carvalho Nunes, H.R. et al. Two-year risedronate treatment for osteoporosis in patients with esophageal varices: a non-randomized clinical trial. Hepatol Int 16, 1458–1467 (2022). https://doi.org/10.1007/s12072-022-10366-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10366-z