Abstract
A green method for synthesizing 1,4-dihydropyridines (1a-j) by a one-pot reaction of various aldehydes, ethyl acetoacetate, and ammonium carbonate, catalyzed by a seashell and kaolin powders, mixed and calcined at 500 and 800 °C, under solvent-free conditions, has been developed. The catalyst was characterized by physicochemical techniques, namely, X-ray diffraction (XRD), electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS). The mixture composed of 20% seashell and 80% Kaolin (Q3-500), calcined at 500 °C, gave the best results (78 to 96% yields). Q3-500 separation from the reacting medium was carried out by filtration and reused several times with a slight decrease in yields. Compared with other conventional methods, the present method is inexpensive and offers advantages, namely: the catalyst is eco-friendly, recyclable, and gives high yields with shorter reaction time.
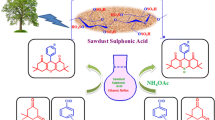
Graphical Abstract
A seashell/Kaolin calcined system (20/80) at 500°C was elaborated to catalyze the Hantzsch reaction under solvent-free conditions. Using Q3-500 six times gave dihydropyridines with excellent yields with a slight loss of activity. The improvement of the catalytic properties of the kaolin surface is due to the increase of cationic content.







Similar content being viewed by others
References
Banerjee S, Horn A, Khatri H and Sereda G 2011 A green one-pot multicomponent synthesis of 4H-pyrans and polysubstituted aniline derivatives of biological, pharmacological, and optical applications using silica nanoparticles as reusable catalyst Tetrahedron Lett. 52 1878
Khaled A, Stiti M Z, Habila T, Ferkhi M, Pirotte B, Pireaux J J and Khelili S 2022 Synthesis and characterization of La1–xSrxMn1-yZnyO3 perovskites as an efficient and recoverable catalyst for the Hantzsch reaction J. Chem. Sci. 134 1
Akika F Z, Kihal N, Habila T, Avramova I, Süzer Ş, Pirotte B and Khelili S 2013 Synthesis and characterization of Zn(1–x)NixAl2O4 spinels as a new heterogeneous catalyst of biginelli’s reaction Bull. Kor. Chem. Soc. 34 1445
Janis R A and Triggle D J 1983 New developments in calcium ion channel antagonists J. Med. Chem. 26 775
Stiti M Z, Belghobsi M, Habila T, Goffin E, De Tullio P, Pirotte B, et al. 2020 Synthesis and vasodilator activity of new 1,4-dihyropyridines bearing sulfonylurea, urea and thiourea moieties Chem. Pap. 74 915
Liang L, Kung J Y, Mitchelmore B, Cave A and Banh H L 2022 Comparative peripheral edema for dihydropyridines calcium channel blockers treatment: A systematic review and network meta-analysis J. Clin. Hypertens. 24 536
Sidhom P A, El-Bastawissy E, Salama A A and El-Moselhy T F 2021 Revisiting ageless antiques; synthesis, biological evaluation, docking simulation and mechanistic insights of 1, 4-Dihydropyridines as anticancer agents Bioorg. Chem. 114 105054
Khoshneviszadeh M, Edraki N, Javidnia K, Alborzi A, Pourabbas B, Mardaneh J and Miri R 2009 Synthesis and biological evaluation of some new 1, 4-dihydropyridines containing different ester substitute and diethyl carbamoyl group as anti-tubercular agents Bioorg. Med. Chem. 17 1579
Milkovic L, Vukovic T, Zarkovic N, Tatzber F, Bisenieks E, Kalme Z, et al. 2018 Antioxidative 1,4-dihydropyridine derivatives modulate oxidative stress and growth of human osteoblast-like cells in vitro Antioxidants 7 123
Nosrati A, Amirnejat S and Javanshir S 2021 Preparation, Antibacterial Activity, and Catalytic Application of Magnetic Graphene Oxide-Fucoidan in the Synthesis of 1, 4-Dihydropyridines and Polyhydroquinolines ChemistryOpen 10 1186
Reddy G M and Camilo A Jr 2020 Biologically active dihydropyridines: an efficient green synthesis, antimicrobial properties, machine aided results and SARs Sustain. Chem. Pharm. 17 100303
Hilgeroth A 2002 Dimeric 4-Aryl-1,4-dihydropyridines: development of a third class of nonpeptidic HIV-1 protease inhibitors Mini. Rev. Med. Chem. 2 235
Hantzsch A 1882 Ueber die synthese pyridinartiger verbindungen aus acetessigäther und aldehydammoniak Liebigs Ann. Chem. 215 1
Sridhar R and Perumal P T 2005 A new protocol to synthesize 1, 4-dihydropyridines by using 3, 4, 5-trifluorobenzeneboronic acid as a catalyst in ionic liquid: synthesis of novel 4-(3-carboxyl-1H-pyrazol-4-yl)-1,4-dihydropyridines Tetrahedron 61 2465
Debache A, Ghalem W, Boulcina R, Belfaitah A, Rhouati S and Carboni B 2009 An efficient one-step synthesis of 1,4-dihydropyridines via a triphenylphosphine-catalyzed three-component Hantzsch reaction under mild conditions Tetrahedron Lett. 50 5248
Wang L M, Sheng J, Zhang L, Han J W, Fan ZY, Tian H and Qian C T 2005 Facile Yb (OTf) 3 promoted one-pot synthesis of polyhydroquinoline derivatives through Hantzsch reaction Tetrahedron 61 1539
Rafiee E, Eavani S, Rashidzadeh S and Joshaghani M 2009 Silica supported 12-tungstophosphoric acid catalysts for synthesis of 1, 4-dihydropyridines under solvent-free conditions Inorg. Chim. Acta 362 3555
Ko S, Sastry M, Lin C and Yao C F 2005 Molecular iodine-catalyzed one-pot synthesis of 4-substituted-1, 4-dihydropyridine derivatives via Hantzsch reaction Tetrahedron Lett. 46 5771
Heravi M M, Bakhtiari K, Javadi N M, Bamoharram F F, Saeedi M and Oskooie H A 2007 K7[PW11CoO40]-catalyzed one-pot synthesis of polyhydroquinoline derivatives via the Hantzsch three component condensation J. Mol. Catal. A 264 50
Yadav D, Patel R, Srivastava V, Watal G and Yadav L 2011 LiBr as an Efficient Catalyst for One-Pot Synthesis of Hantzsch 1, 4-Dihydropyridines under Mild Conditions Chin. J. Chem. 29 118
Ji S J, Jiang Z Q, Lu J and Loh T P 2004 Facile ionic liquids-promoted one-pot synthesis of polyhydroquinoline derivatives under solvent free conditions Synlett 05 0831
Adibi H, Samimi H A and Beygzadeh M 2007 Iron (III) trifluoroacetate and trifluoromethanesulfonate: Recyclable Lewis acid catalysts for one-pot synthesis of 3, 4-dihydropyrimidinones or their sulfur analogues and 1,4-dihydropyridines via solvent-free Biginelli and Hantzsch condensation protocols Catal. Commun. 8 2119
Murthy Y, Rajack A and Ramji M T 2012 Design, solvent free synthesis, and antimicrobial evaluation of 1,4 dihydropyridines Biorg. Med. Chem. Lett. 22 6016
Wang P, Wang J, Au CT, Qiu R, Xu X and Yin S F 2016 Air-stable Organobismuth (V) Bisperfluorooctanesulfonate as an Efficient Catalyst for the Synthesis of N-Containing Compounds Adv. Synth. Catal. 358 1302
Srinivasan V V, Ranoux A, Maheswari R, Hanefeld U, Ramanathan A and Subramaniam B 2016 Potential applications of Zr-KIT-5: Hantzsch reaction, Meerwein–Ponndorf–Verley (MPV) reduction of 4-tert-butylcyclohexanone, and Prins reaction of citronellal Res. Chem. Intermed. 42 2399
Chen C, Lan G and Tuan W 2000 Microstructural evolution of mullite during the sintering of kaolin powder compacts Ceram. Int. 26 715
Hoque M E, Shehryar M and Islam K N 2013 Processing and characterization of cockle shell calcium carbonate (CaCO3) bioceramic for potential application in bone tissue engineering J. Mater. Sci. Eng. 2 132
Jahanbin B, Davoodnia A, Behmadi H and Tavakoli-Hoseini N 2012 Polymer support immobilized acidic ionic liquid: Preparation and its application as catalyst in the synthesis of Hantzsch 1, 4-dihydropyridines Bull. Korean Chem. Soc. 33 2140
Moghaddam F M, Saeidian H, Mirjafary Z and Sadeghi A 2009 Rapid and efficient one-pot synthesis of 1,4-dihydropyridine and polyhydroquinoline derivatives through the Hantzsch four component condensation by zinc oxide J. Iran. Chem. Soc. 6 317
Tajbakhsh M, Alaee E, Alinezhad H, Khanian M, Jahani F, Khaksar S, et al. 2012 Titanium dioxide nanoparticles catalyzed synthesis of Hantzsch esters and polyhydroquinoline derivatives Chin. J. Catal. 33 1517
Debache A, Boulcina R, Belfaitah A, Rhouati S and Carboni B 2008 One-pot synthesis of 1,4-dihydropyridines via a phenylboronic acid catalyzed Hantzsch three-component reaction Synlett 04 509
Srinivasan V V, Pachamuthu M P and Maheswari R 2015 Lewis acidic mesoporous Fe-TUD-1 as catalysts for synthesis of Hantzsch 1,4-dihydropyridine derivatives J. Porous Mater. 22 1187
Ghosh P P, Paul S and Das A R 2013 Light induced synthesis of symmetrical and unsymmetrical dihydropyridines in ethyl lactate–water under tunable conditions Tetrahedron Lett. 54 138
Wang X, Gong H, Quan Z, Li L and Ye H 2011 One-pot, three-component synthesis of 1,4-dihydropyridines in PEG-400 Synth. Commun. 41 3251
Kiyani H and Ghiasi M 2015 Solvent-free efficient one-pot synthesis of Biginelli and Hantzsch compounds catalyzed by potassium phthalimide as a green and reusable organocatalyst Res. Chem. Intermed. 41 5177
Datta B and Pasha M A 2011 Silica sulfuric acid: An efficient heterogeneous catalyst for the one-pot synthesis of 1, 4-dihydropyridines under mild and solvent-free conditions Chin. J. Catal. 32 1180
Acknowledgments
This study was supported by grants from the Algerian Ministry of Higher Education and Scientific Research (PRFU code: B00L01UN180120200002). The authors gratefully acknowledge the technical assistance of M. Aibech Riad. We are also grateful to Mrs Bouharriche Meriem for her language support.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stiti, MZ., Habila, T., Khaled, A. et al. A highly efficient and green method for catalyzing the Hantzsch reaction under solvent-free conditions using a seashell/Kaolin calcined system. J Chem Sci 135, 70 (2023). https://doi.org/10.1007/s12039-023-02190-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-023-02190-1