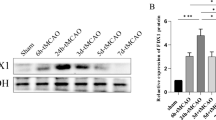
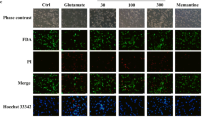
Abstract
Cerebral ischemia-reperfusion injury produces excessive reactive oxygen and nitrogen species, including superoxide, nitric oxide, and peroxynitrite (ONOO-). We recently developed a new ONOO--triggered metal-free carbon monoxide donor (PCOD585), exhibiting a notable neuroprotective outcome on the rat middle cerebral artery occlusion model and rendering an exciting intervention opportunity toward ischemia-induced brain injuries. However, its therapeutic mechanism still needs to be addressed. In the pharmacological study, we found PCOD585 inhibited neuronal Bcl2/Bax/caspase-3 apoptosis pathway in the peri-infarcted area of stroke by scavenging ONOO-. ONOO- scavenging further led to decreased Acyl-CoA synthetase long-chain family member 4 and increased glutathione peroxidase 4, to minimize lipoperoxidation. Additionally, the carbon monoxide release upon the ONOO- reaction with PCOD585 further inhibited the neuronal Iron-dependent ferroptosis associated with ischemia-reperfusion. Such a synergistic neuroprotective mechanism of PCOD585 yields as potent a neuroprotective effect as Edaravone. Additionally, PCOD585 penetrates the blood-brain barrier and reduces the degradation of zonula occludens-1 by inhibiting matrix metalloproteinase-9, thereby protecting the integrity of the blood-brain barrier. Our study provides a new perspective for develo** multi-functional compounds to treat ischemic stroke.







Similar content being viewed by others
Data Availability
The datasets generated and analyzed during this study are available upon reasonable request from the corresponding author.
Abbreviations
- ACSL4:
-
Acyl-CoA synthetase long-chain family member 4
- BBB:
-
Blood-brain barrier
- CO:
-
Carbon monoxide
- CORM-3:
-
Carbon monoxide releasing molecular-3
- DMT1:
-
Divalent metal transporter 1
- EDA:
-
Edaravone
- Fer-1:
-
Ferrostatin-1
- Fpn-1:
-
Ferroportin-1
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GPX4:
-
Glutathione peroxidase 4
- I/R:
-
Ischemia/reperfusion
- MCAO:
-
Middle Cerebral Artery Occlusion
- MMP:
-
Matrix metalloproteinase
- mNSS:
-
Modified Neurological Severity Scores
- OGD/R:
-
Oxygen and Glucose Deprivation/Reperfusion
- PDC:
-
Peroxynitrite decomposition catalyst
- ROS:
-
Reactive oxygen species
- TFR1:
-
Transferrin receptor 1
- TUNEL:
-
TdT-mediated dUTP Nick-End Labeling
References
Zhang Y, Chen S, Tang W (2017) Two-year outcome after endovascular treatment for stroke. N Engl J Med 376(26):2597
Nezu T, Mukai T, Uemura J, Yamashita M, Kitano T, Wada Y et al (2016) Multiple infarcts are associated with long-term stroke recurrence and all-cause mortality in cryptoge nic stroke patients. Stroke 47(9):2209–2215
Salinas J, Sprinkhuizen SM, Ackerson T, Bernhardt J, Davie C, George MG et al (2016) An international standard set of patient-centered outcome measures after stroke. Stroke. 47(1):180–186
Weisenburger-Lile D, Blanc R, Kyheng M, Desilles JP, Labreuche J, Piotin M et al (2019) Direct admission versus secondary transfer for acute stroke patients treated with intravenous thrombo lysis and thrombectomy: insights from the endovascular treatment in ischemic stroke registry. Cerebrovasc Dis 47(3-4):112–120
Chouchani ET, Pell VR, Gaude E, Aksentijevi D, Sundier SY, Robb EL et al (2014) Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 515(7527):431–435
Jiang XC, **ang JJ, Wu HH, Zhang TY, Zhang DP, Xu QH et al (2019) Neural stem cells transfected with reactive oxygen species-responsive polyplexes for effective treatm ent of ischemic stroke. Adv Mater 31(10):e1807591
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317
Morris B (2003) The components of the wired spanning forest are recurrent. Probab Theory Relat Fields 125(2):259–265
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 149(5):1060–1072
Tuo QZ, Lei P, Jackman KA, Li XL, **ong H, Li XL et al (2017) Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry 22(11):1520–1530
Ji C, Steimle BL, Bailey DK, Kosman DJ (2018) The ferroxidase hephaestin but not amyloid precursor protein is required for ferroportin-supported iron efflux in primary hippocampal neurons. Cell Mol Neurobiol 38(4):941–954
Omori N, Maruyama K, ** G, Li F, Wang SJ, Hamakawa Y et al (2003) Targeting of post-ischemic cerebral endothelium in rat by liposomes bearing polyethylene glycol-coupled transferrin. Neurol Res 25(3):275–279
Liu J, Guo ZN, Yan XL, Huang S, Ren JX, Luo Y et al (2020) Crosstalk between autophagy and ferroptosis and its putative role in ischemic stroke. Front Cell Neurosci 14:577403
Sakai O, Yasuzawa T, Sumikawa Y, Ueta T, Imai H, Sawabe A et al (2017) Role of GPx4 in human vascular endothelial cells, and the compensatory activity of brown rice on GPx4 ablation condition. Pathophysiology 24(1):9–15
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171(2):273–285
Chen LD, Wu RH, Huang YZ, Chen MX, Zeng AM, Zhuo GF et al (2020) The role of ferroptosis in chronic intermittent hypoxia-induced liver injury in rats. Sleep Breath 24(4):1767–1773
Yang H, Zhao L, Gao Y, Yao F, Marti TM, Schmid RA et al (2020) Pharmacotranscriptomic analysis reveals novel drugs and gene networks regulating ferroptosis in cancer. Cancers (Basel) 12(11):3273
Wu X, Li Y, Zhang S, Zhou X (2021) Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 11(7):3052–3059
Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E et al (2019) Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177(5):1262–1279.e1225
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156(1-2):317–331
García-Yébenes I, Sobrado M, Moraga A, Zarruk JG, Romera VG, Pradillo JM et al (2012) Iron overload, measured as serum ferritin, increases brain damage induced by focal ischemia and early reperfusion. Neurochem Int 61(8):1364–1369
Park J, Lee DG, Kim B, Park SJ, Kim JH, Lee SR et al (2015) Iron overload triggers mitochondrial fragmentation via calcineurin-sensitive signals in HT-22 hippocampal neuron cells. Toxicology 337:39–46
Wang J, Zhang D, Fu X, Yu L, Lu Z, Gao Y et al (2018) Carbon monoxide-releasing molecule-3 protects against ischemic stroke by suppressing neuroinflammation and alleviating blood-brain barrier disruption. J Neuroinflammation 15(1):188
Basuroy S, Leffler CW, Parfenova H (2013) CORM-A1 prevents blood-brain barrier dysfunction caused by ionotropic glutamate receptor-mediated endothelial oxidative stress and apoptosis. Am J Phys Cell Phys 304(11):C1105–C1115
**ng L, Wang B, Li J, Guo X, Lu X, Chen X et al (2022) A fluorogenic ONOO--triggered carbon monoxide donor for mitigating brain ischemic damage. J Am Chem Soc 144(5):2114–2119
Zhang J, Liu L, Dong Z, Lu X, Hong W, Liu J et al (2023) An ischemic area-targeting, peroxynitrite-responsive, biomimetic carbon monoxide nanogenerator for preventing myocardial ischemia-reperfusion injury. Bioact Mater 28:480–494
Parween S, Alawathugoda TT, Prabakaran AD, Dheen ST, Morse RH, Emerald BS et al (2022) Nutrient sensitive protein O-GlcNAcylation modulates the transcriptome through epigenetic mechanisms during embryonic neurogenesis. Life Sci Alliance 5(8):e202201385
Chen H, Guan B, Chen X, Chen X, Li C, Qiu J et al (2018) Baicalin attenuates blood-brain barrier disruption and hemorrhagic transformation and improves neurol ogical outcome in ischemic stroke rats with delayed t-PA treatment: involvement of ONOO--MMP-9 pathway. Transl Stroke Res 9(5):515–529
Liu Y, Xue X, Zhang H, Che X, Luo J, Wang P et al (2019) Neuronal-targeted TFEB rescues dysfunction of the autophagy-lysosomal pathway and alleviates ischemic injury in permanent cerebral ischemia. Autophagy 15(3):493–509
Michel BW, Lippert AR, Chang CJ (2012) A reaction-based fluorescent probe for selective imaging of carbon monoxide in living cells using a palladium-mediated carbonylation. J Am Chem Soc 134(38):15668–15671
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79(4):1431–1568
Naito MG, Xu D, Amin P, Lee J, Wang H, Li W et al (2020) Sequential activation of necroptosis and apoptosis cooperates to mediate vascular and neural pathology in stroke. P Natl Acad Sci USA 117(9):4959–4970
Miotto G, Rossetto M, Di Paolo ML, Orian L, Venerando R, Roveri A et al (2020) Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol 28:101328
Celi LA, Citi L, Ghassemi M, Pollard TJ (2019) The plos one collection on machine learning in health and biomedicine: towards open code and open data. PLoS One 14(1):e0210232
Halm M (2019) The influence of appropriate staffing and healthy work environments on patient and nurse outcomes. Am J Crit Care 28(2):152–156
Kuo YC, Chen HH (2006) Effect of nanoparticulate polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate on th e permeability of zidovudine and lamivudine across the in vitro blood-brain barrier. Int J Pharm 327(1-2):160–169
Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M et al (2019) Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25(2):270–276
Rubin LL, Staddon JM (1999) The cell biology of the blood-brain barrier. Annu Rev Neurosci 22:11–28
Spronk E, Sykes G, Falcione S, Munsterman D, Joy T, Kamtchum-Tatuene J et al (2021) Hemorrhagic transformation in ischemic stroke and the role of inflammation. Front Neurol 12:661955
Ding R, Feng L, He L, Chen Y, Wen P, Fu Z et al (2015) Peroxynitrite decomposition catalyst prevents matrix metalloproteinase-9 activation and neurovascular injury after hemoglobin injection into the caudate nucleus of rats. Neuroscience. 297:182–193
Basuroy S, Leffler CW, Parfenova H (2013) CORM-A1 prevents blood-brain barrier dysfunction caused by ionotropic glutamate receptor-mediated endothelial oxidative stress and apoptosis. Am J Physiol-Cell Ph 304(11):C1105–C1115
Mao R, Zong N, Hu Y, Chen Y, Xu Y (2022) Neuronal death mechanisms and therapeutic strategy in ischemic stroke. Neurosci Bull 38(10):1229–1247
Yuan J (2009) Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 14(4):469–477
Kang YP, Mockabee-Macias A, Jiang C, Falzone A, Prieto-Farigua N, Stone E et al (2021) Non-canonical glutamate-cysteine ligase activity protects against ferroptosis. Cell Metab 33(1):174–189.e177
Hoy AJ, Nagarajan SR, Butler LM (2021) Tumour fatty acid metabolism in the context of therapy resistance and obesity. Nat Rev Cancer 21(12):753–766
Strzyz P (2020) Iron expulsion by exosomes drives ferroptosis resistance. Nat Rev Mol Cell Biol 21(1):4–5
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H et al (2021) DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 593(7860):586–590
Jang S, Chapa-Dubocq XR, Tyurina YY, St Croix CM, Kapralov AA, Tyurin VA et al (2021) Elucidating the contribution of mitochondrial glutathione to ferroptosis in cardiomyocytes. Redox Biol 45:102021
Gill D, Monori G, Tzoulaki I, Dehghan A (2018) Iron status and risk of stroke. Stroke 49(12):2815–2821
Kaluza J, Wolk A, Larsson SC (2013) Heme iron intake and risk of stroke: a prospective study of men. Stroke 44(2):334–339
García-Yébenes I, García-Culebras A, Peña-Martínez C, Fernández-López D, Díaz-Guzmán J, Negredo P et al (2018) Iron overload exacerbates the risk of hemorrhagic transformation after tPA (tissue-type plasminogen activator) administration in thromboembolic stroke mice. Stroke. 49(9):2163–2172
Kawabata H (2019) Transferrin and transferrin receptors update. Free Radical Bio Med 133:46–54
Yanatori I, Kishi F (2019) DMT1 and iron transport. Free Radical Bio Med 133:55–63
Rishi G, Wallace DF, Subramaniam VN (2015) Hepcidin: regulation of the master iron regulator. Biosci Rep 35(3):e00192
Rishi G, Secondes ES, Wallace DF, Subramaniam VN (2020) Evidence for dimerization of ferroportin in a human hepatic cell line using proximity ligation assays. Biosci Rep 40(5):BSR20191499
Shi J, Yu W, Xu L, Yin N, Liu W, Zhang K et al (2020) Bioinspired nanosponge for salvaging ischemic stroke via free radical scavenging and self-adapted oxy gen regulating. Nano Lett 20(1):780–789
Ren JX, Li C, Yan XL, Qu Y, Yang Y, Guo ZN (2021) Crosstalk between oxidative stress and ferroptosis/oxytosis in ischemic stroke: possible targets and molecular mechanisms. Oxidative Med Cell Longev 2021:6643382
Cheng J, Fan YQ, Liu BH, Zhou H, Wang JM, Chen QX (2020) ACSL4 suppresses glioma cells proliferation via activating ferroptosis. Oncol Rep 43(1):147–158
Chen J, Yang L, Geng L, He J, Chen L, Sun Q et al (2021) Inhibition of acyl-coa synthetase long-chain family member 4 facilitates neurological recovery after stroke by regulation ferroptosis. Front Cell Neurosci 15:632354
Guo J, Cheng M, Liu P, Cao D, Luo J, Wan Y et al (2022) A multi-target directed ligands strategy for the treatment of Alzheimer's disease: dimethyl fumarate plus tranilast modified dithiocarbate as AChE inhibitor and Nrf2 activator. Eur J Med Chem 242:114630
Pereira de Ávila MA, Giusti-Paiva A, Giovani de Oliveira Nascimento C (2014) The peripheral antinociceptive effect induced by the heme oxygenase/carbon monoxide pathway is associated with ATP-sensitive K+ channels. Eur J Pharmacol 726:41–48
Zhang LM, Zhang DX, Zheng WC, Hu JS, Fu L, Li Y et al (2021) CORM-3 exerts a neuroprotective effect in a rodent model of traumatic brain injury via the bidirectional gut-brain interactions. Exp Neurol 341:113683
Soni HM, Jain MR, Mehta AA (2012) Mechanism(s) involved in carbon monoxide-releasing Molecule-2-mediated cardioprotection during ischaemia-reperfusion injury in isolated rat heart. Indian J Pharm Sci 74(4):281–291
Lu K, Wu WJ, Zhang C, Zhu YL, Zhong JQ, Li J (2020) CORM-3 regulates microglia activity, prevents neuronal injury, and improves memory function during radiation-induced brain injury. Curr Neurovasc Res 17(4):464–470
Acknowledgements
We sincerely thank Dr. Fuxing Dong from the Public Experimental Research Center for his enthusiastic help in the experiment of laser scanning confocal microscopy.
Funding
This work was supported by Grants of Natural Science Foundation of Jiangsu Province, China (No. BK20211348), Xuzhou Basic Research Program (KC21030), Leadership program through open competition in Xuzhou Medical University (JBGS202203), Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX21_2722).
Author information
Authors and Affiliations
Contributions
XJG, LYH and SHQ. conceived and designed the research. XJG, LYH and STG contributed equally to this work with the help of ML, WW, JC and YDZ, XG and XL performed immunofluorescence experiments. XC, LL, and YY provided technical assistance with the biological and chemical knowledge. LYH and XJG wrote the manuscript with help from XL All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The animal experiment was conducted by the national and institutional guidelines on ethics and biosafety, and the protocol was approved by the Institutional Animal Care and Use Committee of Xuzhou Medical University (License ID: 201907W079).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
Figure S1. Representative images of Rhodamine B and CO production in different organs from the MCAO rats treated with PCOD585. The cryosections were prepared from the rats subjected to 2 h ischemia and followed with 6 mg/kg PCOD585 intravenous transfusion. The fluorescent images were captured by invert fluorescent microscopy. Rhodamine B (red), CO (Cop-1 probe, green), DAPI (blue). Scale bar: 200 μm. Figure S2. The expression and analysis of GPX4 (green) in caudate putamen from the rats with MCAO, PDC and PCOD585. PDC: MCAO+PDC, PCOD585: MCAO+PCOD585. The data were obtained from three individual experiments. For each experiment, five fluorescent fields were selected. **P < 0. 01, ***P < 0.001. Scale bar: 200 μm. Figure S3. The effect of different reoxygenation times on the expression of GPX4 in OGD-treated HT22 cells. The data were obtained from three individual experiments. *P < 0. 05, **P < 0.01. Figure S4. The effect of PCOD585 with a different concentration on the expression of GPX4 in OGD/R-treated HT22 cells. PCOD585 were added along with reoxygenation. The data were obtained from three individual experiments. *P < 0. 05 (DOCX 1615 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, XJ., Huang, LY., Gong, ST. et al. Peroxynitrite-Triggered Carbon Monoxide Donor Improves Ischemic Stroke Outcome by Inhibiting Neuronal Apoptosis and Ferroptosis. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04238-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04238-w