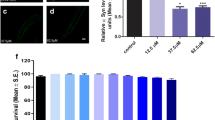
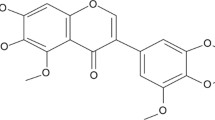
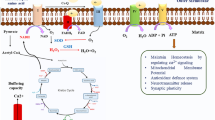
Abstract
Numerous natural antioxidants have been developed into agents for neurodegenerative diseases (NDs) treatment. Rosmarinic acid (RA), an excellent antioxidant, exhibits neuroprotective activity, but its anti-NDs efficacy remains puzzling. Here, Caenorhabditis elegans models were employed to systematically reveal RA-mediated mechanisms in delaying NDs from diverse facets, including oxidative stress, the homeostasis of neural and protein, and mitochondrial disorders. Firstly, RA significantly inhibited reactive oxygen species accumulation, reduced peroxide malonaldehyde production, and strengthened the antioxidant defense system via increasing superoxide dismutase activity. Besides, RA reduced neuronal loss and ameliorated polyglutamine and ɑ-synuclein-mediated dyskinesia in NDs models. Further, in combination with the data and molecular docking results, RA may bind specifically to Huntington protein and ɑ-synuclein to prevent toxic protein aggregation and thus enhance proteostasis. Finally, RA ameliorated mitochondrial dysfunction including increasing adenosine triphosphate and mitochondrial membrane potential levels and rescuing mitochondrial membrane proteins’ expressions and mitochondrial structural abnormalities via regulating mitochondrial dynamics genes and improving the mitochondrial kinetic homeostasis. Thus, this study systematically revealed the RA-mediated neuroprotective mechanism and promoted RA as a promising nutritional intervention strategy to prevent NDs.
Graphical Abstract








Similar content being viewed by others
Data Availability
The date and materials are available from the corresponding author upon reasonable request.
Abbreviations
- ɑ-syn:
-
ɑ-Synuclein
- AD:
-
Alzheimer’s disease
- ATP:
-
Adenosine triphosphate
- CaMKII:
-
II Ca2+/calmodulin-dependent kinase
- DA:
-
Dopaminergic
- DRP-1:
-
Dynamin-related protein 1
- E. coli OP50:
-
Escherichia coli OP50
- GFP:
-
Green fluorescent protein
- HD:
-
Huntington’s disease
- Htt:
-
Huntington protein
- H2DCF-DA:
-
2,7-Dichlorofluorescein diacetate
- HSF-1:
-
Heat-shock factor 1
- IIS:
-
Insulin/insulin-like growth factor 1 signaling
- MDA:
-
Malondialdehyde
- MMP:
-
Mitochondrial membrane potential
- MAPK:
-
Mitogen-activated protein kinases
- NDs:
-
Neurodegenerative diseases
- Nrf2:
-
Mammalian NF-E2-associated factor 2
- OS:
-
Oxidative stress
- OPA1:
-
Optic atrophy 1 protein
- PD:
-
Parkinson’s disease
- polyQ:
-
Polyglutamine
- PHB:
-
Prohibitin
- RA:
-
Rosmarinic acid
- ROS:
-
Reactive oxygen species
- sHSPs:
-
Small heat-shock proteins
- TOM-7:
-
Translocase of the outer mitochondrial membrane 7
- YFP:
-
Yellow fluorescent protein
References
Elfawy HA, Das B (2019) Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: etiologies and therapeutic strategies. Life Sci 218:165–184. https://doi.org/10.1016/j.lfs.2018.12.029
Van Pelt KM, Truttmann MC (2020) Caenorhabditis elegans as a model system for studying aging-associated neurodegenerative diseases. Transl Med Aging 4:60–72. https://doi.org/10.1016/j.tma.2020.05.001
Fan HC, Ho LI, Chi CS, Chen SJ, Peng GS, Chan TM, Lin SZ, Harn HJ (2014) Polyglutamine (PolyQ) diseases: genetics to treatments. Cell Transplant 23(4–5):441–458. https://doi.org/10.3727/096368914X678454
Boasquivis PF, Silva G, Paiva FA, Cavalcanti RM, Nunez CV, Oliveira RD (2018) Guarana (Paullinia cupana) extract protects Caenorhabditis elegans models for Alzheimer disease and Huntington disease through activation of antioxidant and protein degradation pathways. Oxid Med Cell Longev 2018:9241308. https://doi.org/10.1155/2018/9241308
Cooper JF, Van Raamsdonk JM (2018) Modeling Parkinson’s disease in C. elegans. J Parkinson’s Dis 8(1):17–32
Di Rosa G, Brunetti G, Scuto M, Trovato Salinaro A, Calabrese EJ, Crea R, Schmitz-Linneweber C, Calabrese V et al (2020) Healthspan enhancement by olive polyphenols in C. elegans wild type and Parkinson’s models. Int J Mol Sci 21(11):3893. https://doi.org/10.3390/ijms21113893
Amor S, Puentes F, Baker D, Van Der Valk P (2010) Inflammation in neurodegenerative diseases. Immunology 129(2):154–169. https://doi.org/10.1111/j.1365-2567.2009.03225.x
Taylor JP, Hardy J, Fischbeck KH (2002) Toxic proteins in neurodegenerative disease. Science 296(5575):1991–1995. https://doi.org/10.1126/science.1067122
Chung CG, Lee H, Lee SB (2018) Mechanisms of protein toxicity in neurodegenerative diseases. Cell Mol Life Sci 75(17):3159–3180. https://doi.org/10.1007/s00018-018-2854-4
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795. https://doi.org/10.1038/nature05292
Zhao Y, Zhao B (2013) Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longev 2013:1–10. https://doi.org/10.1155/2013/316523
Romero-Márquez JM, Navarro-Hortal MD, Jiménez-Trigo V, Vera-Ramírez L, Forbes-Hernández TJ, Esteban-Muñoz A, Giampieri F, Bullón P et al (2022) An oleuropein rich-olive (Olea europaea L.) leaf extract reduces β-amyloid and tau proteotoxicity through regulation of oxidative- and heat shock-stress responses in Caenorhabditis elegans. Food Chem Toxicol 162:112914
Silva RFM, Pogačnik L (2020) Polyphenols from food and natural products: neuroprotection and safety. Antioxidants (Basel) 9(1):61. https://doi.org/10.3390/antiox9010061
Dhouafli Z, Cuanalo-Contreras K, Hayouni EA, Mays CE, Soto C, Moreno-Gonzalez I (2018) Inhibition of protein misfolding and aggregation by natural phenolic compounds. Cell Mol Life Sci 75(19):3521–3538. https://doi.org/10.1007/s00018-018-2872-2
Yamamoto S, Kayama T, Noguchi-Shinohara M, Hamaguchi T, Yamada M, Abe K, Kobayashi S (2021) Rosmarinic acid suppresses tau phosphorylation and cognitive decline by downregulating the JNK signaling pathway. NPJ Sci Food 5(1):1. https://doi.org/10.1038/s41538-021-00084-5
Lin C, **ao J, ** Y, Zhang X, Zhong Q, Zheng H, Cao Y, Chen Y (2019) Rosmarinic acid improved antioxidant properties and healthspan via the IIS and MAPK pathways in Caenorhabditis elegans. BioFactors 45(5):774–787. https://doi.org/10.1002/biof.1536
Lee HJ, Cho H, Park E, Kim S, Lee S, Kim C, Kim DK, Kim S et al (2008) Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology 250(2–3):109–115. https://doi.org/10.1016/j.tox.2008.06.010
Thingore C, Kshirsagar V, Juvekar A (2021) Amelioration of oxidative stress and neuroinflammation in lipopolysaccharide-induced memory impairment using Rosmarinic acid in mice. Metab Brain Dis 36(2):299–313. https://doi.org/10.1007/s11011-020-00629-9
Wang J, Xu H, Jiang H, Du X, Sun P, **e J (2012) Neurorescue effect of rosmarinic acid on 6-hydroxydopamine-lesioned nigral dopamine neurons in rat model of Parkinson’s disease. J Mol Neurosci 47(1):113–119. https://doi.org/10.1007/s12031-011-9693-1
Markaki M, Tavernarakis N (2020) Caenorhabditis elegans as a model system for human diseases. Curr Opin Biotechnol 63:118–125. https://doi.org/10.1016/j.copbio.2019.12.011
Vaccaro A, Tauffenberger A, Aggad D, Rouleau G, Drapeau P, Parker JA (2012) Mutant TDP-43 and FUS cause age-dependent paralysis and neurodegeneration in C. elegans. PLoS ONE 7(2):e31321. https://doi.org/10.1371/journal.pone.0031321
Dostal V, Link CD (2010) Assaying β-amyloid toxicity using a transgenic C. elegans model. J Vis Exp 9(44):e2252
Ma X, Li J, Cui X, Li F, Wang Z (2019) Dietary supplementation with peptides from sesame cake protect Caenorhabditis elegans from polyglutamine-induced toxicity. J Funct Foods 54:199–210. https://doi.org/10.1016/j.jff.2019.01.002
Morley JF, Brignull HR, Weyers JJ, Morimoto RI (2002) The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci 99(16):10417–10422. https://doi.org/10.1073/pnas.152161099
Chen Y, Qin Q, Zhao W, Luo D, Huang Y, Liu G, Kuang Y, Cao Y et al (2022) Carnosol reduced pathogenic protein aggregation and cognitive impairment in neurodegenerative diseases models via improving proteostasis and ameliorating mitochondrial disorders. J Agric Food Chem 70(34):10490–10505. https://doi.org/10.1021/acs.jafc.2c02665
Zeng XS, Geng WS, Jia JJ (2018) Neurotoxin-induced animal models of Parkinson disease: pathogenic mechanism and assessment. ASN Neuro 10:1663354718. https://doi.org/10.1177/1759091418777438
Tucci ML, Harrington AJ, Caldwell GA, Caldwell KA (2011) Modeling dopamine neuron degeneration in Caenorhabditis elegans. Methods Mol Biol 793:129–148. https://doi.org/10.1007/978-1-61779-328-8_9
Muhammad F, Liu Y, Wang N, Zhao L, Zhou Y, Yang H, Li H (2022) Anti-synuclein toxicity and anti-neurodegenerative role of chrysin in transgenic Caenorhabditis elegans models of Parkinson’s disease. ACS Chem Neurosci 13(4):442–453. https://doi.org/10.1021/acschemneuro.1c00548
Roussel N, Sprenger J, Tappan SJ, Glaser JR (2015) Robust tracking and quantification of C. elegans body shape and locomotion through coiling, entanglement, and omega bends. Worm 3(4):e982437. https://doi.org/10.4161/21624054.2014.982437
Sun J, Roy S (2021) Gene-based therapies for neurodegenerative diseases. Nat Neurosci 24(3):297–311. https://doi.org/10.1038/s41593-020-00778-1
Pohl F, Kong TLP (2018) The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: in vitro, in vivo and clinical trials. Molecules 23(12):3283. https://doi.org/10.3390/molecules23123283
Chia SJ, Tan E, Chao Y (2020) Historical perspective: models of Parkinson’s disease. Int J Mol Sci 21(7):2464. https://doi.org/10.3390/ijms21072464
Khanna A, Sellegounder D, Kumar J, Chamoli M, Vargas M, Chinta SJ, Rane A, Nelson C et al (2021) Trimethylamine modulates dauer formation, neurodegeneration, and lifespan throughtyra-3/daf-11 signaling in Caenorhabditis elegans. Aging Cell 20(5):e13351. https://doi.org/10.1111/acel.13351
Rekatsina M, Paladini A, Piroli A, Zis P, Pergolizzi JV, Varrassi G (2020) Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: a narrative review. Adv Ther 37(1):113–139. https://doi.org/10.1007/s12325-019-01148-5
Sanyal I, Bandyopadhyay SK, Banerjee TK, Mukherjee SC, Chakraborty DP, Ray BC, Rao VR (2009) Plasma levels of lipid peroxides in patients with Parkinson’s disease. Eur Rev Med Pharmacol Sci 13(2):129–132
Dib M, Garrel C, Favier A, Robin V, Desnuelle C (2002) Can malondialdehyde be used as a biological marker of progression in neurodegenerative disease? J Neurol 249(4):367–374. https://doi.org/10.1007/s004150200025
Ayala A, Muñoz MF, Argüelles S, Ramana KV (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:360431–360438. https://doi.org/10.1155/2014/360438
Chen CM, Wu YR, Cheng ML, Liu JL, Lee YM, Lee PW, Soong BW, Chiu DT (2007) Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington’s disease patients. Biochem Biophys Res Commun 359(2):335–340. https://doi.org/10.1016/j.bbrc.2007.05.093
Schuessel K, Schäfer S, Bayer TA, Czech C, Pradier L, Müller-Spahn F, Müller WE, Eckert A (2005) Impaired Cu/Zn-SOD activity contributes to increased oxidative damage in APP transgenic mice. Neurobiol Dis 18(1):89–99. https://doi.org/10.1016/j.nbd.2004.09.003
Chi H, Chang H, Sang T (2018) Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci 19(10):3082. https://doi.org/10.3390/ijms19103082
Anjaneyulu J, R V, Godbole A (2020) Differential effect of Ayurvedic nootropics on C. elegans models of Parkinson’s disease. J Ayurveda Integr Med 11(4):440–447. https://doi.org/10.1016/j.jaim.2020.07.006
Cordeiro LM, Machado ML, Da SA, Obetine BF, Da ST, Soares F, Arantes LP (2020) Rutin protects Huntington’s disease through the insulin/IGF1 (IIS) signaling pathway and autophagy activity: Study in Caenorhabditis elegans model. Food Chem Toxicol 141:111323. https://doi.org/10.1016/j.fct.2020.111323
Inestrosa NC, Varela-Nallar L (2014) Wnt signaling in the nervous system and in Alzheimer’s disease. J Mol Cell Biol 6(1):64–74. https://doi.org/10.1093/jmcb/mjt051
Rosso SB, Inestrosa NC (2013) WNT signaling in neuronal maturation and synaptogenesis. Front Cell Neurosci 7:103. https://doi.org/10.3389/fncel.2013.00103
Maro GS, Klassen MP, Shen K (2009) A β-catenin-dependent Wnt pathway mediates anteroposterior axon guidance in C. elegans motor neurons. PLoS ONE 4(3):e4690. https://doi.org/10.1371/journal.pone.0004690
Serafino A, Sferrazza G, Colini Baldeschi A, Nicotera G, Andreola F, Pittaluga E, Pierimarchi P (2016) Develo** drugs that target the Wnt pathway: recent approaches in cancer and neurodegenerative diseases. Expert Opin Drug Discov 12(2):169–186. https://doi.org/10.1080/17460441.2017.1271321
Purro SA, Galli S, Salinas PC (2014) Dysfunction of Wnt signaling and synaptic disassembly in neurodegenerative diseases. J Mol Cell Biol 6(1):75–80. https://doi.org/10.1093/jmcb/mjt049
Gao J, Liao Y, Qiu M, Shen W (2021) Wnt/beta-catenin signaling in neural stem cell homeostasis and neurological diseases. Neuroscientist 27(1):58–72. https://doi.org/10.1177/1073858420914509
Arredondo SB, Valenzuela-Bezanilla D, Mardones MD, Varela-Nallar L (2020) Role of Wnt signaling in adult hippocampal neurogenesis in health and disease. Front Cell Dev Biol 8:860. https://doi.org/10.3389/fcell.2020.00860
Maleki Dana P, Sadoughi F, Mansournia MA, Mirzaei H, Asemi Z, Yousefi B (2021) Targeting Wnt signaling pathway by polyphenols: implication for aging and age-related diseases. Biogerontology (Dordrecht) 22(5):479–494. https://doi.org/10.1007/s10522-021-09934-x
Inestrosa NC, Toledo EM (2008) The role of Wnt signaling in neuronal dysfunction in Alzheimer’s disease. Mol Neurodegener 3(1):9. https://doi.org/10.1186/1750-1326-3-9
Kapoor ANDA (2020) Role of Notch signaling in neurovascular aging and Alzheimer’s disease. Seminars in Cell & Developmental Biology Elsevier. doi:https://doi.org/10.1016/j.semcdb.2020.12.011
Woo H, Park J, Gwon A, Arumugam TV, Jo D (2009) Alzheimer’s disease and Notch signaling. Biochem Biophys Res Commun 390(4):1093–1097. https://doi.org/10.1016/j.bbrc.2009.10.093
Son HG, Altintas O, Kim EJE, Kwon S, Lee SJV (2019) Age-dependent changes and biomarkers of aging in Caenorhabditis elegans. Aging Cell 18(2):e12853. https://doi.org/10.1111/acel.12853
Alves LGA, Winter PB, Ferreira LN, Brielmann RM, Morimoto RI, Amaral LAN (2017) Long-range correlations and fractal dynamics in C. elegans: Changes with aging and stress. Phys Rev E 96(2):022417. https://doi.org/10.1103/PhysRevE.96.022417
Munoz-Lobato F, Rodriguez-Palero MJ, Naranjo-Galindo FJ, Shephard F, Gaffney CJ, Szewczyk NJ, Hamamichi S, Caldwell KA et al (2014) Protective role of DNJ-27 / ERdj5 in Caenorhabditis elegans models of human neurodegenerative diseases. Antioxid Redox Signal 20(2):217–235. https://doi.org/10.1089/ars.2012.5051
Burke KA, Hensal KM, Umbaugh CS, Chaibva M, Legleiter J (2013) Huntingtin disrupts lipid bilayers in a polyQ-length dependent manner. Biochimica et Biophysica Acta (BBA)-Biomembrane 1828(8):1953–1961. https://doi.org/10.1016/j.bbamem.2013.04.025
Bose A, Beal MF (2016) Mitochondrial dysfunction in Parkinson’s disease. J Neurochem 139(S1):216–231. https://doi.org/10.1111/jnc.13731
Kotler SA, Tugarinov V, Schmidt T, Ceccon A, Libich DS, Ghirlando R, Schwieters CD, Clore GM (2019) Probing initial transient oligomerization events facilitating Huntingtin fibril nucleation at atomic resolution by relaxation-based NMR. Proc Natl Acad Sci U S A 116(9):3562–3571. https://doi.org/10.1073/pnas.1821216116
Salveson PJ, Spencer RK, Nowick JS (2016) X-ray crystallographic structure of oligomers formed by a toxic β-hairpin derived from α-synuclein: trimers and higher-order oligomers. J Am Chem Soc 138(13):4458–4467. https://doi.org/10.1021/jacs.5b13261
Yamashita H, Tomoshige S, Nomura S, Ohgane K, Hashimoto Y, Ishikawa M (2020) Application of protein knockdown strategy targeting β-sheet structure to multiple disease-associated polyglutamine proteins. Bioorg Med Chem 28(1):115175. https://doi.org/10.1016/j.bmc.2019.115175
Fujiwara K, Toda H, Ikeguchi M (2012) Dependence of alpha-helical and beta-sheet amino acid propensities on the overall protein fold type. BMC Struct Biol 12(1):18. https://doi.org/10.1186/1472-6807-12-18
Wanker EE (2000) Protein aggregation in Huntington’s and Parkinson’s disease: implications for therapy. Mol Med Today 6(10):387–391. https://doi.org/10.1016/S1357-4310(00)01761-5
Cohen E, Dillin A (2008) The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci 9(10):759–767. https://doi.org/10.1038/nrn2474
Knight AL, Yan X, Hamamichi S, Ajjuri RR, Mazzulli JR, Zhang MW, Daigle JG, Zhang S et al (2014) The glycolytic enzyme, GPI, is a functionally conserved modifier of dopaminergic neurodegeneration in Parkinson’s models. Cell Metab 20(1):145–157. https://doi.org/10.1016/j.cmet.2014.04.017
Mukhopadhyay A, Oh SW, Tissenbaum HA (2006) Worming pathways to and from DAF-16/FOXO. Exp Gerontol 41(10):928–934. https://doi.org/10.1016/j.exger.2006.05.020
Zhang L, Jie G, Zhang J, Zhao B (2009) Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic Biol Med 46(3):414–421. https://doi.org/10.1016/j.freeradbiomed.2008.10.041
Kourtis N, Nikoletopoulou V, Tavernarakis N (2012) Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature 490(7419):213–218. https://doi.org/10.1038/nature11417
Koga H, Kaushik S, Cuervo AM (2011) Protein homeostasis and aging: the importance of exquisite quality control. Ageing Res Rev 10(2):205–215. https://doi.org/10.1016/j.arr.2010.02.001
Neef DW, Jaeger AM, Thiele DJ (2011) Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat Rev Drug Discov 10(12):930–944. https://doi.org/10.1038/nrd3453
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475(7356):324–332. https://doi.org/10.1038/nature10317
Govindan S, Amirthalingam M, Duraisamy K, Govindhan T, Sundararaj N, Palanisamy S (2018) Phytochemicals-induced hormesis protects Caenorhabditis elegans against alpha-synuclein protein aggregation and stress through modulating HSF-1 and SKN-1/Nrf2 signaling pathways. Biomed Pharmacother 102:812–822. https://doi.org/10.1016/j.biopha.2018.03.128
Fonte V, Kipp DR, Yerg JR, Merin D, Forrestal M, Wagner E, Roberts CM, Link CD (2008) Suppression of in vivo beta-amyloid peptide toxicity by overexpression of the HSP-16.2 small chaperone protein. J Biol Chem 283(2):784–791. https://doi.org/10.1074/jbc.M703339200
Corrêa SAL, Eales KL (2012) The role of p38 MAPK and its substrates in neuronal plasticity and neurodegenerative disease. J Signal Transduction 2012:1–12. https://doi.org/10.1155/2012/649079
Yu CW, Wei CC, Liao VHC (2013) Curcumin-mediated oxidative stress resistance in Caenorhabditis elegans is modulated byage-1, akt-1, pdk-1, osr-1, unc-43, sek-1, skn-1, sir-2.1, and mev-1. Free Radic Res 48(3):371–379. https://doi.org/10.3109/10715762.2013.872779
Pietsch K, Saul N, Menzel R, Stürzenbaum SR, Steinberg CEW (2009) Quercetin mediated lifespan extension in Caenorhabditis elegans is modulated by age-1, daf-2, sek-1 and unc-43. Biogerontology 10(5):565–578. https://doi.org/10.1007/s10522-008-9199-6
Jodeiri Farshbaf M, Ghaedi K (2017) Huntington’s disease and mitochondria. Neurotox Res 32(3):518–529. https://doi.org/10.1007/s12640-017-9766-1
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu S (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287(4):C817–C833. https://doi.org/10.1152/ajpcell.00139.2004
Wang X, Su B, Zheng L, Perry G, Smith MA, Zhu X (2009) The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Neurochem 109(s1):153–159. https://doi.org/10.1111/j.1471-4159.2009.05867.x
Waizenegger T, Schmitt S, Zivkovic J, Neupert W, Rapaport D (2005) Mim1, a protein required for the assembly of the TOM complex of mitochondria. EMBO Rep 6(1):57–62. https://doi.org/10.1038/sj.embor.7400318
Acín-Pérez R, Fernández-Silva P, Peleato ML, Pérez-Martos A, Enriquez JA (2008) Respiratory active mitochondrial supercomplexes. Mol Cell 32(4):529–539. https://doi.org/10.1016/j.molcel.2008.10.021
Signorile A, Sgaramella G, Bellomo F, De Rasmo D (2019) Prohibitins: a critical role in mitochondrial functions and implication in diseases. Cells (Basel, Switzerland) 8(1):71. https://doi.org/10.3390/cells8010071
Merkwirth C, Martinelli P, Korwitz A, Morbin M, Brönneke HS, Jordan SD, Rugarli EI, Langer T (2012) Loss of prohibitin membrane scaffolds impairs mitochondrial architecture and leads to tau hyperphosphorylation and neurodegeneration. PLoS Genet 8(11):e1003021. https://doi.org/10.1371/journal.pgen.1003021
Ye J, Li J, **a R, Zhou M, Yu L (2015) Prohibitin protects proximal tubule epithelial cells against oxidative injury through mitochondrial pathways. Free Radic Res 49(11):1393–1403. https://doi.org/10.3109/10715762.2015.1075654
Lee H, Smith SB, Yoon Y (2017) The short variant of the mitochondrial dynamin OPA1 maintains mitochondrial energetics and cristae structure. J Biol Chem 292(17):7115–7130. https://doi.org/10.1074/jbc.M116.762567
Palmer CS, Osellame LD, Stojanovski D, Ryan MT (2011) The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal 23(10):1534–1545. https://doi.org/10.1016/j.cellsig.2011.05.021
Byrne JJ, Soh MS, Chandhok G, Vijayaraghavan T, Teoh J, Crawford S, Cobham AE, Yapa NMB et al (2019) Disruption of mitochondrial dynamics affects behaviour and lifespan in Caenorhabditis elegans. Cell Mol Life Sci 76(10):1967–1985. https://doi.org/10.1007/s00018-019-03024-5
Starr DA, Han M (2002) Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science 298(5592):406–409. https://doi.org/10.1126/science.1075119
Tsang WY, Lemire BD (2003) Mitochondrial ATP synthase controls larval development cell nonautonomously in Caenorhabditis elegans. Dev Dyn 226(4):719–726. https://doi.org/10.1002/dvdy.10272
Acknowledgements
C. elegans strains were provided by the Caenorhabditis Genetics Center at University of Minnesota.
Funding
This research was supported by the General project of the Natural Science Foundation of Guangdong Province, China (2022A1515010907; 2023A1515011266) and the Action for Revitalizing Seed Industry and Promoting Agriculture through Science and Technology (2023LZ04).
Author information
Authors and Affiliations
Contributions
Yun.C.: formal analysis, investigation, writing-original draft, writing-review and editing, data curation. R.X.: investigation, data curation, writing-review and editing. Q.L.: investigation, writing-original draft, visualization, data curation. Y.Z.: project administration, visualization, investigation. W.C.: data curation, visualization, investigation. Y.L.: project administration, visualization, investigation. Y.Cao: conceptualization, writing-review and editing. G.L.: methodology, writing-review and editing, conception. Yunj.C.: conceptualization, methodology, writing-review and editing, funding acquisition.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yun Chen and Ruina Xu should be considered joint first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Xu, R., Liu, Q. et al. Rosmarinic acid ameliorated oxidative stress, neuronal injuries, and mitochondrial dysfunctions mediated by polyglutamine and ɑ-synuclein in Caenorhabditis elegans models. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04206-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04206-4