Abstract
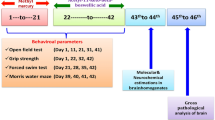
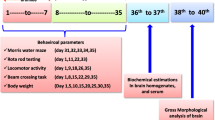
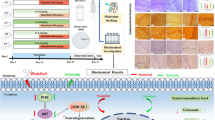
Amyotrophic lateral sclerosis (ALS) is a paralytic disease that damages the brain and spinal cord motor neurons. Several clinical and preclinical studies have found that methylmercury (MeHg+) causes ALS. In ALS, MeHg+-induced neurotoxicity manifests as oligodendrocyte destruction; myelin basic protein (MBP) deficiency leads to axonal death. ALS development has been connected to an increase in signal transducer and activator of transcription-3 (STAT-3), a mammalian target of rapamycin (mTOR), and a decrease in peroxisome proliferator-activated receptor (PPAR)-gamma. Guggulsterone (GST), a plant-derived chemical produced from Commiphorawhighitii resin, has been found to protect against ALS by modulating these signaling pathways. Vitamin D3 (VitD3) deficiency has been related to oligodendrocyte precursor cells (OPC) damage, demyelination, and white matter deterioration, which results in motor neuron death. As a result, the primary goal of this work was to investigate the therapeutic potential of GST by altering STAT-3, mTOR, and PPAR-gamma levels in a MeHg+-exposed experimental model of ALS in adult rats. The GST30 and 60 mg/kg oral treatments significantly improved the behavioral, motor, and cognitive dysfunctions and increased remyelination, as proven by the Luxol Fast Blue stain (LFB), and reduced neuroinflammation as measured by histological examinations. Furthermore, the co-administration of VitD3 exhibits moderate efficacy when administered in combination with GST60. Our results show that GST protects neurons by decreasing STAT-3 and mTOR levels while increasing PPAR-gamma protein levels in ALS rats.



























Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article. There are no separate or additional files.
Abbreviations
- AAS:
-
Atomic absorption spectroscopy
- Ach:
-
Acetylcholine
- AchE:
-
Acetylcholinesterase
- ALS:
-
Amyotrophic lateral sclerosis
- ALT:
-
Alanine transaminase
- ANOVA:
-
Analysis of variance
- Bax:
-
Bcl-2 associated X protein
- Bcl-2:
-
B cell lymphoma-2
- BDNF:
-
Brain-derived growth factor
- cAMP:
-
Cyclic AMP
- Caspase-3:
-
Cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate directed proteases-3
- CAT:
-
Catalase
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- ELT:
-
Escape latency time
- FST:
-
Forced swim test
- GABA:
-
Gamma amino butyric acid
- GSH:
-
Reduced glutathione
- GST:
-
Guggulsterone
- IL-1β:
-
Interleukin-1β
- JAK:
-
Janus kinase
- LFB:
-
Luxol Fast Blue
- MBP:
-
Myelin basic protein
- MDA:
-
Malondialdehyde
- MeHg:
-
Methylmercury
- mTOR:
-
Mammalian target of rapamycin
- MND:
-
Motor neuron disease
- MWM:
-
Morris water maze
- NEFL:
-
Neurofilament
- NGF:
-
Nerve growth factor
- NO2 :
-
Nitrite
- ODC:
-
Oligodendrocytes
- OPC:
-
Oligodendrocyte precursor cells
- PPAR-gamma:
-
Peroxisome proliferator-activated receptors
- ROS:
-
Reactive oxygen species
- STAT:
-
Signal transducer and activator of transcription
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor-α
- TSTQ:
-
Time spent in target quadrant
- 5-HT:
-
Serotonin
- VitD3 :
-
Vitamin D3
References
Minj E, Upadhayay S, Mehan S (2021) Nrf2/HO-1 signaling activator acetyl-11-keto-beta Boswellic acid (AKBA)-mediated neuroprotection in methyl mercury-induced experimental model of ALS. Neurochem Res 46(11):2867–2884. https://doi.org/10.1007/s11064-021-03366-2
Sahu R, Upadhayay S, Mehan S (2021) Inhibition of extracellular regulated kinase (ERK)-1/2 signaling pathway in the prevention of ALS: target inhibitors and influences on neurological dysfunctions. Eur J Cell Biol 100(7–8):151179
Alam MM, Minj E, Yadav RK, Mehan S (2021) Neuroprotective potential of adenyl cyclase/cAMP/CREB and mitochondrial CoQ10 activator in amyotrophic lateral sclerosis rats. Curr Bioact Compd 17(5):53–69
Shandilya A, Mehan S (2021) Dysregulation of IGF-1/GLP-1 signaling in the progression of ALS: potential target activators and influences on neurological dysfunctions. Neurol Sci 42(8):3145–3166
Kühnlein P, Gdynia HJ, Sperfeld AD, Lindner-Pfleghar B, Ludolph AC, Prosiegel M, Riecker A (2008) Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat Clin Pract Neurol 4(7):366–374. https://doi.org/10.1038/ncpneuro0853
Castoldi AF, Johansson C, Onishchenko N, Coccini T, Roda E, Vahter M, ... Manzo L (2008) Human developmental neurotoxicity of methylmercury: impact of variables and risk modifiers. Regul Toxicol Pharmacol 51(2):201–214
Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, ... Peineau S (2013) The role of JAK-STAT signaling within the CNS. Jak-stat 2(1):e22925
Sharma A, Mehan S (2021) Targeting PI3K-AKT/mTOR signaling in the prevention of autism. Neurochem Int 147:105067
Sharma A, Bhalla S, Mehan S (2022) PI3K/AKT/mTOR signalling inhibitor chrysophanol ameliorates neurobehavioural and neurochemical defects in propionic acid-induced experimental model of autism in adult rats. Metab Brain Dis 37(6):1909–1929. https://doi.org/10.1007/s11011-022-01026-0
Kumar S, Mehan S, Narula AS (2023) Therapeutic modulation of JAK-STAT, mTOR, and PPAR-γ signaling in neurological dysfunctions. J Mol Med (Berl) 101(1–2):9–49. https://doi.org/10.1007/s00109-022-02272-6
**n P, Xu X, Deng C, Liu S, Wang Y, Zhou X, ... Sun S (2020) The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol 80:106210
Zhang Z, Chen F, Li J, Luo F, Hou T, Xu J, Sun D (2018) 1, 25 (OH) 2D3 suppresses proinflammatory responses by inhibiting Th1 cell differentiation and cytokine production through the JAK/STAT pathway. Am J Transl Res 10(8):2737
Yu JH, Kim KH, Kim H (2008) SOCS 3 and PPAR-γ ligands inhibit the expression of IL-6 and TGF-β1 by regulating JAK2/STAT3 signaling in pancreas. Int J Biochem Cell Biol 40(4):677–688
Kumar N, Sharma N, Mehan S (2021) Connection between JAK/STAT and PPARγ signaling during the progression of multiple sclerosis: insights into the modulation of T-cells and immune responses in the brain. Curr Mol Pharmacol 14(5):823–837
Khera R, Mehan S, Kumar S, Sethi P, Bhalla S, Prajapati A (2022) Role of JAK-STAT and PPAR-Gamma signalling modulators in the prevention of autism and neurological dysfunctions. Mol Neurobiol 59(6):3888–3912. https://doi.org/10.1007/s12035-022-02819-1
Vitale G, Zappavigna S, Marra M, Dicitore A, Meschini S, Condello M, ... Caraglia M (2012) The PPAR-γ agonist troglitazone antagonizes survival pathways induced by STAT-3 in recombinant interferon-β treated pancreatic cancer cells. Biotechnol Adv 30(1):169–184. https://doi.org/10.1097/WNR.0000000000001149
Limor R, Sharon O, Knoll E, Many A, Weisinger G, Stern N (2008) Lipoxygenase-derived metabolites are regulators of peroxisome proliferator-activated receptor γ-2 expression in human vascular smooth muscle cells. Am J Hypertens 21(2):219–223
Zhang L, Fang Y, Cheng X, Lian Y, Xu H, Zeng Z, Zhu H (2017) TRPML1 participates in the progression of Alzheimer’s disease by regulating the PPARγ/AMPK/Mtorsignalling pathway. Cell Physiol Biochem 43(6):2446–2456
Kumar N, Sahoo NK, Mehan S, Verma B (2023) The importance of gut-brain axis and use of probiotics as a treatment strategy for multiple sclerosis. Mult Scler Relat Disord 71:104547. Advance online publication. https://doi.org/10.1016/j.msard.2023.104547
Zhao JL, Wei C, **ao X, Dong YH, Tan B, Yu J, ... **e R (2020) Expression of TNF-α and IL-β can be suppressed via the PPAR-γ/mTOR signaling pathway in BV-2 microglia: a potential anti-inflammation mechanism. Mol Med Rep 22(4):3559–3565
Yadav RK, Mehan S, Sahu R, Kumar S, Khan A, Makeen HA, Al Bratty M (2022) Protective effects of apigenin on methylmercury-induced behavioral/neurochemical abnormalities and neurotoxicity in rats. Hum Exp Toxicol 41:09603271221084276
Sahu R, Mehan S, Kumar S, Prajapati A, Alshammari A, Alharbi M, ... Narula AS (2022) Effect of alpha-mangostin in the prevention of behavioural and neurochemical defects in methylmercury-induced neurotoxicity in experimental rats. Toxicol Rep 9:977–998
Bhalla S, Mehan S (2022) 4-hydroxyisoleucine mediated IGF-1/GLP-1 signalling activation prevents propionic acid-induced autism-like behavioural phenotypes and neurochemical defects in experimental rats. Neuropeptides 96:102296. https://doi.org/10.1016/j.npep.2022.102296
Khera H, Awasthi A, Mehan S (2019) Myocardial preconditioning potential of hedgehog activator purmorphamine (smoothened receptor agonist) against ischemia-reperfusion in deoxycortisone acetate salt-induced hypertensive rat hearts. J Pharmacol Pharmacother 10(2):47–56
Saxena G, Singh SP, Pal R, Singh S, Pratap R, Nath C (2007) Gugulipid, an extract of Commiphorawhighitii with lipid-lowering properties, has protective effects against streptozotocin-induced memory deficits in mice. Pharmacol Biochem Behav 86(4):797–805. https://doi.org/10.1016/j.pbb.2007.03.010
Liu FG, Hu WF, Wang JL, Wang P, Gong Y, Tong LJ, ... Huang C (2017) Z-guggulsterone produces antidepressant-like effects in mice through activation of the BDNF signaling pathway. Int J Neuropsychopharmacol 20(6):485–497
Muthian G, Raikwar HP, Rajasingh J, Bright JJ (2006) 1, 25 dihydroxyvitamin-D3 modulates JAK–STAT pathway in IL-12/IFNγ axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res 83(7):1299–1309
Hoepner R, Bagnoud M, Pistor M, Salmen A, Briner M, Synn H, ... Chan A (2019) Vitamin D increases glucocorticoid efficacy via inhibition of mTORC1 in experimental models of multiple sclerosis. Acta Neuropathol 138(3):443–456
Li DW, Ren H, Jeromin A, Liu M, Shen D, Tai H et al (2018) Diagnostic performance of neurofilaments in Chinese patients with amyotrophic lateral sclerosis: a prospective study. Front Neurol 9:726. https://doi.org/10.3389/fneur.2018.00726
Sato Y, Honda Y, Asoh T, Kikuyama M, Oizumi K (1997) Hypovitaminosis D and decreased bone mineral density in amyotrophic lateral sclerosis. Eur Neurol 37(4):225
Mashayekhi F, Salehi Z (2016) Administration of vitamin D 3 induces CNPase and myelin oligodendrocyte glycoprotein expression in the cerebral cortex of the murine model of cuprizone-induced demyelination. Folia Neuropathol 54(3):259–264
Gomez-Pinedo U, Cuevas JA, Benito-Martín MS, Moreno-Jiménez L, Esteban-Garcia N, Torre-Fuentes L, Matías-Guiu JA, Pytel V et al (2020) Vitamin D increases remyelination by promoting oligodendrocyte lineage differentiation. Brain Behav 10(1):e01498. https://doi.org/10.1002/brb3.1498
Mengozzi M, Hesketh A, Bucca G, Ghezzi P, Smith CP (2020) Vitamins D3 and D2 have marked but different global effects on gene expression in a rat oligodendrocyte precursor cell line. Mol Med 26(1):32. https://doi.org/10.1186/s10020-020-00153-7
Rajdev K, Siddiqui EM, Jadaun KS, Mehan S (2020) Neuroprotective potential of solanesol in a combined model of intracerebral and intraventricular hemorrhage in rats. IBRO Rep 8:101–114
Gupta R, Mehan S, Sethi P, Prajapati A, Alshammari A, Alharbi M, ... Narula AS (2022) Smo-Shh agonist Purmorphamine prevents neurobehavioral and neurochemical defects in 8-OH-DPAT-induced experimental model of obsessive-compulsive disorder. Brain Sci 12(3):342
Duggal P, Jadaun KS, Siqqiqui EM, Mehan S (2020) Investigation of low dose cabazitaxel potential as microtubule stabilizer in experimental model of Alzheimer’s disease: restoring neuronal cytoskeleton. Curr Alzheimer Res 17(7):601–615
Rajkhowa B, Mehan S, Sethi P, Prajapati A, Suri M, Kumar S, Bhalla S, Narula AS et al (2022) Activating SIRT-1 signalling with the mitochondrial-CoQ10 activator solanesol improves neurobehavioral and neurochemical defects in Ouabain-induced experimental model of bipolar disorder. Pharmaceuticals (Basel, Switzerland) 15(8):959. https://doi.org/10.3390/ph15080959
Verma L, Sakir M, Singh N, Mehra R, Mehan S (2010) Development of phase change solutions for ophthalmic drug delivery based on ion activated and pH induced polymers. Int J Pharm Prof Res 1(2):127–134
Shandilya A, Mehan S, Kumar S, Sethi P, Narula AS, Alshammari A, ... Alasmari AF (2022) Activation of IGF-1/GLP-1 signalling via 4-hydroxyisoleucine prevents motor neuron impairments in experimental ALS-rats exposed to methylmercury-induced neurotoxicity. Molecules 27(12):3878
Yasutake A, Nagano M, Nakano A (2005) Simple method for methylmercury estimation in biological samples using atomic absorption spectroscopy. J Health Sci 51(2):220–223
Gao D, ** N, Fu Y, Zhu Y, Wang Y, Wang T, ... Li Y (2021) Rational drug design of benzothiazole-based derivatives as potent signal transducer and activator of transcription 3 (STAT3) signaling pathway inhibitors. Eur J Med Chem 216:113333
Jadaun KS, Mehan S, Sharma A, Siddiqui EM, Kumar S, Alsuhaymi N (2022) Neuroprotective effect of chrysophanol as a PI3K/AKT/mTOR signaling inhibitor in an experimental model of autologous blood induced Intracerebral Hemorrhage. Curr Med Sci 42(2):249–266. https://doi.org/10.1007/s11596-022-2496-x
Cui J, Cui C, Cui Y, Li R, Sheng H, Jiang X, ... Gao J (2017) Bone marrow mesenchymal stem cell transplantation increases GAP-43 expression via ERK1/2 and PI3K/Akt pathways in intracerebral hemorrhage. Cell Physiol Biochem 42(1):137–144
Duelsner A, Gatzke N, Hillmeister P, Glaser J, Zietzer A, Nagorka S, ... Buschmann IR (2014) PPAR γ activation inhibits cerebral arteriogenesis in the hypoperfused rat brain. Acta Physiol 210(2):354–368
Tiwari A, Khera R, Rahi S, Mehan S, Makeen HA, Khormi YH, ..., Khan A (2021) Neuroprotective effect of α-mangostin in ameliorating propionic acid-induced experimental model of autism in Wistar rats. Brain Sci 11(3):288
Alharbi M, Alshammari A, Kaur G, Kalra S, Mehan S, Suri M, Chhabra S, Kumar N et al (2022) Effect of natural adenylcyclase/cAMP/CREB signalling activator forskolin against intra-striatal 6-OHDA-lesioned Parkinson’s rats: preventing mitochondrial, motor and histopathological defects. Molecules (Basel, Switzerland) 27(22):7951. https://doi.org/10.3390/molecules27227951
González-Fraguela ME, Hung MLD, Vera H, Maragoto C, Noris E, Blanco L, ... Robinson M (2013) Oxidative stress markers in children with autism spectrum disorders. Br J Med Med Res 3(2):307
Kapoor T, Mehan S, Suri M, Sharma N, Kumar N, Narula AS, Alshammari A, Alasmari AF et al (2022) Forskolin, an adenylcyclase/cAMP/CREB signaling activator restoring myelin-associated oligodendrocyte destruction in experimental ethidium bromide model of multiple sclerosis. Cells 11(18):2771. https://doi.org/10.3390/cells11182771
Singh A, Upadhayay S, Mehan S (2021) Understanding abnormal c-JNK/p38MAPK signaling overactivation involved in the progression of multiple sclerosis: possible therapeutic targets and impact on neurodegenerative diseases. Neurotox Res 39(5):1630–1650
Bala R, Khanna D, Mehan S, Kalra S (2015) Experimental evidence for the potential of lycopene in the management of scopolamine induced amnesia. RSC Adv 5(89):72881–72892
Sharma R, Rahi S, Mehan S (2019) Neuroprotective potential of solanesol in intracerebroventricular propionic acid induced experimental model of autism: insights from behavioral and biochemical evidence. Toxicol Rep 6:1164–1175
Speaker KJ, Cox SS, Paton MM, Serebrakian A, Maslanik T, Greenwood BN, Fleshner M (2014) Six weeks of voluntary wheel running modulates inflammatory protein (MCP-1, IL-6, and IL-10) and DAMP (Hsp72) responses to acute stress in white adipose tissue of lean rats. Brain Behav Immun 39:87–98
Wu N, Shen H, Liu H, Wang Y, Bai Y, Han P (2016) Acute blood glucose fluctuation enhances rat aorta endothelial cell apoptosis, oxidative stress and pro-inflammatory cytokine expression in vivo. Cardiovasc Diabetol 15(1):1–13
Chen F, Wang W, Ding H, Yang Q, Dong Q, Cui M (2016) The glucagon-like peptide-1 receptor agonist exendin-4 ameliorates warfarin-associated hemorrhagic transformation after cerebral ischemia. J Neuroinflammation 13(1):1–14
Singh L, Rana S, Mehan S (2018) Role of adenylyl cyclase activator in controlling experimental diabetic nephropathy in rats. Int J Physiol Pathophysiol Pharmacol 10(5):144
Mehan S, Rahi S, Tiwari A, Kapoor T, Rajdev K, Sharma R, ... Dudi R (2020) Adenylate cyclase activator forskolin alleviates intracerebroventricular propionic acid-induced mitochondrial dysfunction of autistic rats. Neural Regener Res 15(6):1140
Deshmukh R, Sharma V, Mehan S, Sharma N, Bedi KL (2009) Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine—a PDE1 inhibitor. Eur J Pharmacol 620(1–3):49–56
Albekairi TH, Kamra A, Bhardwaj S, Mehan S, Giri A, Suri M, Alshammari A, Alharbi M et al (2022) Beta-boswellic acid reverses 3-nitropropionic acid-induced molecular, mitochondrial, and histopathological defects in experimental rat model of Huntington’s disease. Biomedicines 10(11):2866. https://doi.org/10.3390/biomedicines10112866
Siddiqui EM, Mehan S, Upadhayay S, Khan A, Halawi M, Halawi AA, Alsaffar RM (2021) Neuroprotective efficacy of 4-hydroxyisoleucine in experimentally induced intracerebral hemorrhage. Saudi J Biol Sci 28(11):6417–6431
Zerwekh JE (2008) Blood biomarkers of vitamin D status. Am J Clin Nutr 87(4):1087S-1091S
Mori N, Yasutake A, Marumoto M, Hirayama K (2011) Methylmercury inhibits electron transport chain activity and induces cytochrome c release in cerebellum mitochondria. J Toxicol Sci 36(3):253–259
Khan Z, Gupta GD, Mehan S (2023) Cellular and molecular evidence of multiple sclerosis diagnosis and treatment challenges. J Clin Med 12(13):4274. Published 2023 Jun 26. https://doi.org/10.3390/jcm12134274
Vallée A, Vallée JN, Guillevin R, Lecarpentier Y (2018) Interactions between the canonical WNT/beta-catenin pathway and PPAR gamma on neuroinflammation, demyelination, and remyelination in multiple sclerosis. Cell Mol Neurobiol 38(4):783–795
Park EJ, Park SY, Joe EH, Jou I (2003) 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J Biol Chem 278(17):14747–14752
Krawczyk-Marc I, Wawrzyniak A, Luszczewska-Sierakowska I, Babicz MA, Orkisz ST (2019) Oligodendrocytes: morphology, functions and involvement in neurodegenerative diseases. Med Weter 75(8):465–471
Fields RD (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31(7):361–370
Chiò A, Pagani M, Agosta F, Calvo A, Cistaro A, Filippi M (2014) Neuroimaging in amyotrophic lateral sclerosis: insights into structural and functional changes. Lancet Neurol 13(12):1228–1240
Lorente Pons A, Higginbottom A, Cooper-Knock J, Alrafiah A, Alofi E, Kirby J, Shaw PJ, Wood JD et al (2020) Oligodendrocyte pathology exceeds axonal pathology in white matter in human amyotrophic lateral sclerosis. J Pathol 251(3):262–271
Wang Y, Qin ZH (2010) Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis 15(11):1382–1402
Dong W, Ma Y, Guan F, Zhang X, Chen W, Zhang L, Zhang L (2021) Ablation of C9orf72 together with excitotoxicity induces ALS in rats. FEBS J 288(5):1712–1723
Gyawali A, Kang YS (2021) Transport alteration of 4-phenyl butyric acid mediated by a sodium-and proton-coupled monocarboxylic acid transporter system in ALS model cell lines (NSC-34) under inflammatory states. J Pharm Sci 110(3):1374–1384
Foerster BR, Pomper MG, Callaghan BC, Petrou M, Edden RA, Mohamed MA, Welsh RC, Carlos RC et al (2013) An imbalance between excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis revealed by use of 3-T proton magnetic resonance spectroscopy. JAMA Neurol 70(8):1009–1016
Lu CH, Allen K, Oei F, Leoni E, Kuhle J, Tree T, Fratta P, Sharma N, Sidle K, Howard R, Orrell R, Fish M, Greensmith L, Pearce N, Gallo V, Malaspina A (2016) Systemic inflammatory response and neuromuscular involvement in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm 3(4):e244. https://doi.org/10.1212/NXI.0000000000000244
Babu GN, Kumar A, Chandra R, Puri SK, Kalita J, Misra UK (2008) Elevated inflammatory markers in a group of amyotrophic lateral sclerosis patients from northern India. Neurochem Res 33(6):1145–1149
Iwai-Shimada M, Takahashi T, Kim MS, Fujimura M, Ito H, Toyama T, Naganuma A, Hwang GW (2016) Methylmercury induces the expression of TNF-α selectively in the brain of mice. Sci Rep 6(1):1–8
Dangoumau A, Marouillat S, Coelho R, Wurmser F, Brulard C, Haouari S, Laumonnier F, Corcia P et al (2021) Dysregulations of expression of genes of the ubiquitin/sumo pathways in an in vitro model of amyotrophic lateral sclerosis combining oxidative stress and sod1 gene mutation. Int J Mol Sci 22(4):1796
Zuo X, Zhou J, Li Y, Wu K, Chen Z, Luo Z, Zhang X, Liang Y et al (2021) TDP-43 aggregation induced by oxidative stress causes global mitochondrial imbalance in ALS. Nat Struct Mol Biol 28(2):132–142
Zucchi E, Bonetto V, Sorarù G, Martinelli I, Parchi P, Liguori R, Mandrioli J (2020) Neurofilaments in motor neuron disorders: towards promising diagnostic and prognostic biomarkers. Mol Neurodegener 15(1):1–20
Campos-Melo D, Hawley ZCE, Strong MJ (2018) Dysregulation of human NEFM and NEFH mRNA stability by ALS-linked miRNAs. Mol Brain 11:43. https://doi.org/10.1186/s13041-018-0386-3
Gagliardi D, Meneri M, Saccomanno D, Bresolin N, Comi GP, Corti S (2019) Diagnostic and prognostic role of blood and cerebrospinal fluid and blood neurofilaments in amyotrophic lateral sclerosis: a review of the literature. Int J Mol Sci 20:4152. https://doi.org/10.3390/ijms20174152
Li X, Xu S, Liu J, Zhao Y, Han H, Li X, Wang Y (2023) Treatment with 1,25-Dihydroxyvitamin D3 delays choroid plexus infiltration and BCSFB injury in MRL/lpr mice coinciding with activation of the PPARγ/NF-κB/TNF-α pathway and suppression of TGF-β/Smad signaling. Inflammation 46(2):556–572. https://doi.org/10.1007/s10753-022-01755-5
Brettschneider J, Petzold A, Süssmuth SD, Ludolph AC, Tumani H (2006) Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 66:852–856. https://doi.org/10.1212/01.wnl.0000203120.85850.54
Gaiottino J, Norgren N, Dobson R, Top** J, Nissim A, Malaspina A et al (2013) Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE 8:e75091. https://doi.org/10.1371/journal.pone.0075091
Cote F, Collard JF, Julien JP (1993) Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Cell 73:35–46. https://doi.org/10.1016/0092-8674(93)90158-M
Xu Z, Cork LC, Griffin JW, Cleveland DW (1993) Increased expression of neurofilament subunit NF-L produces morphological alterations that resemblethe pathology of human motor neuron disease. Cell 73:23–33. https://doi.org/10.1016/0092-8674(93)90157-L
Solomon JA, Gianforcaro A, Hamadeh MJ (2011) Vitamin D3 deficiency differentially affects functional and disease outcomes in the G93A mouse model of amyotrophic lateral sclerosis. PLoS ONE 6(12):e29354
Mohammed HO, Divers TJ, Summers BA, de Lahunta A (2007) Vitamin E deficiency and risk of equine motor neuron disease. Acta Vet Scand 49(1):1–9
Karam C, Barrett MJ, Imperato T, MacGowan DJ, Scelsa S (2013) Vitamin D deficiency and its supplementation in patients with amyotrophic lateral sclerosis. J Clin Neurosci 20(11):1550–1553
Camu W, Tremblier B, Plassot C et al (2014) Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol Aging. 35:1198–1205
Gianforcaro A, Hamadeh MJ (2012) Dietary vitamin D3 supplementation at 10× the adequate intake improves functional capacity in the G93A transgenic mouse model of ALS, a pilot study. CNS Neurosci Ther 18(7):547–557
Farina M, Rocha JB, Aschner M (2011) Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci 89(15–16):555–563
Abrahams S, Goldstein LH, Suckling J, Ng V, Simmons A, Chitnis X, Atkins L, Williams SC et al (2005) Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol 252(3):321–323
Tan RH, Kril JJ, McGinley C, Hassani M, Masuda-Suzukake M, Hasegawa M, Mito R, Kiernan MC et al (2016) Cerebellar neuronal loss in amyotrophic lateral sclerosis cases with ATXN2 intermediate repeat expansions. Ann Neurol 79(2):295–305. https://doi.org/10.1002/ana.24565
Prell T, Grosskreutz J (2013) The involvement of the cerebellum in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 14(7–8):507–515
Grant RA, Sharp PS, Kennerley AJ, Berwick J, Grierson A, Ramesh T, Prescott TJ (2014) Abnormalities in whisking behaviour are associated with lesions in brain stem nuclei in a mouse model of amyotrophic lateral sclerosis. Behav Brain Res 259:274–283
Mekhail M, Almazan G, Tabrizian M (2012) Oligodendrocyte-protection and remyelination post-spinal cord injuries: a review. Prog Neurobiol 96(3):322–339
Piaton G, Gould RM, Lubetzki C (2010) Axon-oligodendrocyte interactions during developmental myelination, demyelination and repair. J Neurochem 114(5):1243–1260
Prajapati A, Mehan S, Khan Z (2023) The role of Smo-Shh/Gli signaling activation in the prevention of neurological and ageing disorders. Biogerontology 24(4):493–531. https://doi.org/10.1007/s10522-023-10034-1
Raffaele S, Boccazzi M, Fumagalli M (2021) Oligodendrocyte dysfunction in amyotrophic lateral sclerosis: mechanisms and therapeutic perspectives. Cells 10(3):565
Khan Z, Gupta GD, Mehan S (2023) Cellular and molecular evidence of multiple sclerosis diagnosis and treatment challenges. J Clin Med 12(13):4274. https://doi.org/10.3390/jcm12134274
Dudi R, Mehan S (2018) Neuroprotection of brain permeable Forskolin ameliorates behavioral, biochemical and histopathological alterations in rat model of intracerebral hemorrhage. Pharmaspire 10(2):68–86. https://isfcppharmaspire.com/uploads/228/13863_pdf.pdf
Acknowledgements
The authors thank Chairman Mr. Parveen Garg, ISF College of Pharmacy, Moga (Punjab), India, for their excellent vision and support.
Funding
This work was supported by DST-SERB, Govt. of India (Grant Number: CRG/2021/001009). This work was supported by institutional grants from the Institutional Animal Ethics Committee (IAEC) with registration no. 816/PO/ReBiBt8/S/04/CPCSEA as protocol no. ISFCP/IAEC/CPCSEA/Meeting No: 30/2021/Protocol No. 505 approved by RAB Committee, ISFCP, Moga, Punjab, India.
Author information
Authors and Affiliations
Contributions
Investigation, original draft, formal analysis, S.K; revision, editing, visualization, G.D.G., Z.K., A.S.N.; conceptualization, resources, supervision, S.M. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of this work, ensuring integrity and accuracy. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
All applicable institutional guidelines for the care and use of animals were followed.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Mehan, S., Khan, Z. et al. Guggulsterone Selectively Modulates STAT-3, mTOR, and PPAR-Gamma Signaling in a Methylmercury-Exposed Experimental Neurotoxicity: Evidence from CSF, Blood Plasma, and Brain Samples. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-023-03902-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03902-x