Abstract
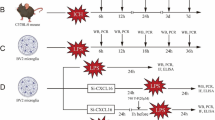
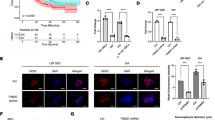
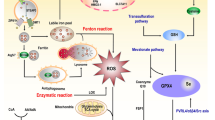
Radiotherapy for head and neck tumors can lead to a severe complication known as radiation-induced brain injury (RIBI). However, the underlying mechanism of RIBI development remains unclear, and limited prevention and treatment options are available. Neuroactive steroids have shown potential in treating neurological disorders. 5α-Androst-3β, 5, 6β-triol (TRIOL), a synthetic neuroprotective steroid, holds promise as a treatment candidate for RIBI patients. However, the neuroprotective effects and underlying mechanism of TRIOL on RIBI treatment are yet to be elucidated. In the present study, our findings demonstrate TRIOL’s potential as a neuroprotective agent against RIBI. In gamma knife irradiation mouse model, TRIOL treatment significantly reduced brain necrosis volume, microglial activation, and neuronal loss. RNA-sequencing, immunofluorescence, real-time quantitative polymerase chain reaction, siRNA transfection, and western blotting techniques revealed that TRIOL effectively decreased microglial activation, proinflammatory cytokine release, neuron loss, and guanylate-binding protein 5 (GBP5) expression, along with its downstream signaling pathways NF-κB and NLRP3 activation in vitro. In summary, TRIOL effectively alleviate RIBI by inhibiting the GBP5/NF-κB/NLRP3 signal axis, reducing microglia activation and pro-inflammation cytokines release, rescuing neuron loss. This study highlights the potential of TRIOL as a novel and promising therapy drug for RIBI treatment.
Graphical Abstract








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Gupta B, Johnson NW, Kumar N (2016) Global epidemiology of head and neck cancers: a continuing challenge. Oncology 91(1):13–23. https://doi.org/10.1159/000446117
Rube CE, Raid S, Palm J, Rube C (2023) Radiation-induced brain injury: age dependency of neurocognitive dysfunction following radiotherapy. Cancers (Basel) 15(11):2999. https://doi.org/10.3390/cancers15112999
Wilke C, Grosshans D, Duman J, Brown P, Li J (2018) Radiation-induced cognitive toxicity: pathophysiology and interventions to reduce toxicity in adults. Neuro Oncol 20(5):597–607. https://doi.org/10.1093/neuonc/nox195
Lin X, Tang L, Li M, Wang M, Guo Z, Lv X, Qiu Y (2021) Irradiation-related longitudinal white matter atrophy underlies cognitive impairment in patients with nasopharyngeal carcinoma. Brain Imaging Behav 15(5):2426–2435
Chang YQ, Zhou GJ, Wen HM, He DQ, Xu CL, Chen YR, Li YH, Chen SX et al (2023) Treatment of radiation-induced brain injury with bisdemethoxycurcumin. Neural Regen Res 18(2):416–421. https://doi.org/10.4103/1673-5374.346549
Yang X, Ren H, Fu JJOM, Longevity C (2021) Treatment of radiation-induced brain necrosis. Oxid Med Cell Longev 2021:4793517
Zheng B, Lin J, Li Y, Zhuo X, Huang X, Shen Q, Tang Y (2019) Predictors of the therapeutic effect of corticosteroids on radiation-induced optic neuropathy following nasopharyngeal carcinoma. Support Care Cancer 27:4213–4219
Xu Y, Rong X, Hu W, Huang X, Li Y, Zheng D, Cai Z et al (2018) Bevacizumab monotherapy reduces radiation-induced brain necrosis in nasopharyngeal carcinoma patients: a randomized controlled trial. Int J Radiat Oncol Biol Phys 101(5):1087–1095
Vaios EJ, Batich KA, Buckley AF, Dunn-Pirio A, Patel MP, Kirkpatrick JP, Goudar R, Peters KB (2022) Resolution of radiation necrosis with bevacizumab following radiation therapy for primary CNS lymphoma. Oncotarget 13:576–582
Li Y, Huang X, Jiang J, Hu W, Hu J, Cai J, Rong X, Cheng J et al (2018) Clinical variables for prediction of the therapeutic effects of bevacizumab monotherapy in nasopharyngeal carcinoma patients with radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 100(3):621–629. https://doi.org/10.1016/j.ijrobp.2017.11.023
Voss M, Wenger KJ, Fokas E, Forster M-T, Steinbach JP, Ronellenfitsch MW (2021) Single-shot bevacizumab for cerebral radiation injury. BMC Neurol 21:1–7
Turnquist C, Harris BT, Harris CC (2020) Radiation-induced brain injury: current concepts and therapeutic strategies targeting neuroinflammation. Neuriincol Adv 2(1):vdaa057
Lumniczky K, Szatmári T, Sáfrány G (2017) Ionizing radiation-induced immune and inflammatory reactions in the brain. Front Immunol 8:517. https://doi.org/10.3389/fimmu.2017.00517
Luo N, Zhu W, Li X, Fu M, Peng X, Yang F, Zhang Y, Yin H et al (2022) Impact of gut microbiota on radiation-associated cognitive dysfunction and neuroinflammation in mice. Radiat Res 197(4):350–364
Liu Q, Huang Y, Duan M, Yang Q, Ren B, Tang F (2022) Microglia as therapeutic target for radiation-induced brain injury. Int J Mol Sci 23(15):8286
Balentova S, Adamkov M (2015) Molecular, cellular and functional effects of radiation-induced brain injury: a review. Int J Mol Sci 16(11):27796–27815
Wang J, Pan H, Lin Z, **ong C, Wei C, Li H, Tong F, Dong X (2021) Neuroprotective effect of fractalkine on radiation-induced brain injury through promoting the M2 polarization of microglia. Mol Neurobiol 58:1074–1087
Zhang Z, Jiang J, He Y, Cai J, **e J, Wu M, **ng M, Zhang Z, Chang H et al (2022) Pregabalin mitigates microglial activation and neuronal injury by inhibiting HMGB1 signaling pathway in radiation-induced brain injury. J Neuroinflammation 19(1):231. https://doi.org/10.1186/s12974-022-02596-7
He B, Wang X, He Y, Li H, Yang Y, Shi Z, Liu Q, Wu M et al (2020) Gamma ray-induced glial activation and neuronal loss occur before the delayed onset of brain necrosis. Faseb j 34(10):13361–13375. https://doi.org/10.1096/fj.202000365RR
Li X, Chen X, Chen J, Zhou S, Wen J, Huang Y, Yan G, Zhang JJ (2015) Synthesis and neuroprotection of 5α-androst-3β, 5, 6β-triol derivatives. Ther Targets Neurol Dis 2:e831
Sun HJ, Xue DD, Lu BZ, Li Y, Sheng LX, Zhu Z, Zhou YW, Zhang JX, Lin GJ, Lin SZ, Yan GM, Chen YP, Yin W (2019) A novel synthetic steroid of 2β,3α,5α-trihydroxy-androst-6-one alleviates the loss of rat retinal ganglion cells caused by acute intraocular hypertension via inhibiting the inflammatory activation of microglia. Molecules 24(2):252. https://doi.org/10.3390/molecules24020252
Xue D, Wei C, Zhou Y, Wang K, Zhou Y, Chen C, Li Y, Sheng L et al (2022) TRIOL inhibits rapid intracellular acidification and cerebral ischemic injury: the role of glutamate in neuronal metabolic reprogramming. ACS Chem Neurosci 13(14):2110–2121. https://doi.org/10.1021/acschemneuro.2c00119
Kutsch M, Coers J (2021) Human guanylate binding proteins: nanomachines orchestrating host defense. FEBS J 288(20):5826–5849. https://doi.org/10.1111/febs.15662
Liu P, Ye L, Ren Y, Zhao G, Zhang Y, Lu S, Li Q, Wu C et al (2023) Chemotherapy-induced phlebitis via the GBP5/NLRP3 inflammasome axis and the therapeutic effect of aescin. Br J Pharmacol 180(8):1132–1147. https://doi.org/10.1111/bph.16002
Zhou L, Zhao H, Zhao H, Meng X, Zhao Z, **e H, Li J, Tang Y et al (2023) GBP5 exacerbates rosacea-like skin inflammation by skewing macrophage polarization towards M1 phenotype through the NF-κB signalling pathway. J Europ Acad Dermatol Venereol: JEADV 37(4):796–809. https://doi.org/10.1111/jdv.18725
Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD (2012) GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336(6080):481–485. https://doi.org/10.1126/science.1217141
Tang L, Wang Y, Leng T, Sun H, Zhou Y, Zhu W, Qiu P, Zhang J et al (2015) Cholesterol metabolite cholestane-3beta,5alpha,6beta-triol suppresses epileptic seizures by negative modulation of voltage-gated sodium channels. Steroids 98:166–172. https://doi.org/10.1016/j.steroids.2014.12.025
Zhang P, Chen JS, Li QY, Sheng LX, Gao YX, Lu BZ, Zhu WB, Zhan XY et al (2020) Neuroprotectants attenuate hypobaric hypoxia-induced brain injuries in cynomolgus monkeys. Zool Res 41(1):3–19. https://doi.org/10.24272/j.issn.2095-8137.2020.012
Tang L, Yan M, Leng T, Yin W, Cai S, Duan S, Zhu W, Lin S et al (2018) Cholestane-3beta, 5alpha, 6beta-triol suppresses neuronal hyperexcitability via binding to voltage-gated sodium channels. Biochem Biophys Res Commun 496(1):95–100. https://doi.org/10.1016/j.bbrc.2018.01.004
Shi Z, Yu P, Lin WJ, Chen S, Hu X, Chen S, Cheng J, Liu Q et al (2023) Microglia drive transient insult-induced brain injury by chemotactic recruitment of CD8(+) T lymphocytes. Neuron 111(5):696-710e 699. https://doi.org/10.1016/j.neuron.2022.12.009
Sun HJ, Xue DD, Lu BZ, Li Y, Sheng LX, Zhu Z, Zhou YW, et al. (2019) A novel synthetic steroid of 2beta,3alpha,5alpha-trihydroxy-androst-6-one alleviates the loss of rat retinal ganglion cells caused by acute intraocular hypertension via inhibiting the inflammatory activation of microglia. Molecules 24(2):252. https://doi.org/10.3390/molecules24020252
Jeyaretna DS, Curry WT Jr, Batchelor TT, Stemmer-Rachamimov A, Plotkin SR (2011) Exacerbation of cerebral radiation necrosis by bevacizumab. J Clin Oncol: Off J Amer Soc Clin Oncol 29(7):e159-162. https://doi.org/10.1200/jco.2010.31.4815
Li Q, Barres BA (2018) Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 18(4):225–242
Kim S, Chung H, Ngoc Mai H, Nam Y, Shin SJ, Park YH, Chung MJ, Lee JK et al (2020) Low-dose ionizing radiation modulates microglia phenotypes in the models of Alzheimer’s disease. Int J Mol Sci 21(12):4532
Chen J, Leng T, Chen W, Yan M, Yin W, Huang Y, Lin S, Duan D et al (2013) A synthetic steroid 5alpha-androst-3beta,5,6beta-triol blocks hypoxia/reoxygenation-induced neuronal injuries via protection of mitochondrial function. Steroids 78(10):996–1002. https://doi.org/10.1016/j.steroids.2013.06.004
Tang L, Yan M, Leng T, Yin W, Cai S, Duan S, Zhu W, Lin S et al (2018) Cholestane-3β, 5α, 6β-triol suppresses neuronal hyperexcitability via binding to voltage-gated sodium channels. Biochem Biophys Res Commun 496(1):95–100. https://doi.org/10.1016/j.bbrc.2018.01.004
Yan M, Leng T, Tang L, Zheng X, Lu B, Li Y, Sheng L, Lin S et al (2017) Neuroprotectant androst-3β, 5α, 6β-triol suppresses TNF-α-induced endothelial adhesion molecules expression and neutrophil adhesion to endothelial cells by attenuation of CYLD-NF-κB pathway. Biochem Biophys Res Commun 483(2):892–896. https://doi.org/10.1016/j.bbrc.2017.01.030
Hu H, Zhou Y, Leng T, Liu A, Wang Y, You X, Chen J, Tang L et al (2014) The major cholesterol metabolite cholestane-3β,5α,6β-triol functions as an endogenous neuroprotectant. J Neurosci: Off J Soc Neurosci 34(34):11426–11438. https://doi.org/10.1523/jneurosci.0344-14.2014
Wang J, Pan H, Lin Z, **ong C, Wei C, Li H, Tong F, Dong X (2021) Neuroprotective effect of fractalkine on radiation-induced brain injury through promoting the M2 polarization of microglia. Mol Neurobiol 58(3):1074–1087. https://doi.org/10.1007/s12035-020-02138-3
Xu Y, Hu W, Liu Y, Xu P, Li Z, Wu R, Shi X, Tang Y (2016) P2Y6 receptor-mediated microglial phagocytosis in radiation-induced brain injury. Mol Neurobiol 53(6):3552–3564. https://doi.org/10.1007/s12035-015-9282-3
Jenrow KA, Brown SL, Lapanowski K, Naei H, Kolozsvary A, Kim JH (2013) Selective inhibition of microglia-mediated neuroinflammation mitigates radiation-induced cognitive impairment. Radiat Res 179(5):549–556. https://doi.org/10.1667/RR3026.1
Boyd A, Byrne S, Middleton RJ, Banati RB, Liu GJ (2021) Control of neuroinflammation through radiation-induced microglial changes. Cells 10(9):2381
Li Y, Lin X, Wang W, Wang W, Cheng S, Huang Y, Zou Y, Ke J et al (2022) The proinflammatory role of guanylate-binding protein 5 in inflammatory bowel diseases. Front Microbiol 13:926915. https://doi.org/10.3389/fmicb.2022.926915
Acknowledgements
We thank Guangzhou Cellprotek Pharmaceutical Co. Ltd who provide the TRIOL.
Funding
This study was supported by the National Natural Science Foundation of China (no. 81925031 and 81820108026), Guangzhou Science and Technology Program Key Projects (202007030001), and STI 2030 Major Projects (2022ZD0211600) to Y.T.; National Natural Science Foundation of China (no. 82103775) and China Postdoctoral Science Foundation (2022M723589) to Z.S.; and Guang Dong Basic and Applied Basic Research Foundation (2022A1515110189) to K.Z.
Author information
Authors and Affiliations
Contributions
ZK designed this study, performed the experiments, and drafted the manuscript. LKJ and SY performed the experiments, carried out extra data analysis, and revised the manuscript. CST performed high-throughput sequencing analysis. HX and XRQ assisted in gamma ray irradiation and MR imaging. MXY, LSJ, and YJW assisted in tissue processing and IF experiments. ZXQ and YMJ assisted in drug administration of animals. HYJ and YW revised the manuscript. CYP, TYM, and SZS revised the manuscript, and supervised the design of the study and conceived the manuscript. All authors reviewed and approved the final version of this paper.
Corresponding authors
Ethics declarations
Ethics Approval
This study was approved by the Ethics Committee of Sun Yat-sen University (SYXK 2022–0289).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12035_2023_3831_MOESM2_ESM.tif
Supplementary file2 (TIF 11774 KB) TRIOL alleviated radiation-induced myelin loss and astrocyte activation. (A) Representative images of MAG immunofluorescent staining in the thalamus of sham mice and two irradiated mice at 8 weeks post irradiation. Red: MAG, blue: DAPI. Quantification of the average fluorescence intensity number of MAG in the thalamus from immunofluorescent images. (B) Representative images of GFAP immunofluorescent staining in thalamus from sham mice and two irradiated mice at 8 weeks post irradiation. Red: GFAP, blue: DAPI. Quantification of the number of GFAP+ cells in the thalamus sections. n= 5 mice per group. Data were analyzed by one-way ANOVA followed by Tukey's post hoc analysis. All other groups were compared with the control group. Scale bar = 50 μm. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, K., Liu, K., Song, Y. et al. A Synthetic Steroid 5α-Androst-3β, 5, 6β-triol Alleviates Radiation-Induced Brain Injury in Mice via Inhibiting GBP5/NF-κB/NLRP3 Signal Axis. Mol Neurobiol (2023). https://doi.org/10.1007/s12035-023-03831-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03831-9