Abstract
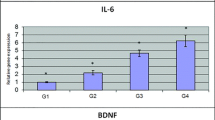
We monitored CSF (cerebrospinal fluid) for Th1/Th2 inflammatory cytokines in a patient with unexplained postoperative disturbance of consciousness after craniotomy and found that the level of IL-6 (interleukin-6) concentrations was extremely high, meeting the traditional criteria for an inflammatory cytokine storm. Subsequently, the cerebrospinal fluid specimens of several patients were tested, and it was found that IL-6 levels were increased in different degrees after craniotomy. Previous studies have focused more on mild and long-term IL-6 elevation, but less on the effects of this short-term IL-6 inflammatory cytokine storm. Cerebrospinal fluid rich in IL-6 may play a significant role in patients after craniotomy. The objective is to explore the degree of IL-6 elevation and the incidence of IL-6 inflammatory cytokine storm in patients after craniotomy, as well as the effect of IL-6 elevation on the brain. In this study, the levels and clinical manifestations of inflammatory factors in cerebrospinal fluid after craniotomy were statistically classified, and the underlying mechanisms were discussed preliminarily. CSF specimens of patients after craniotomy were collected, IL-6 level was measured at 1, 5, and 10 days after operation, and cognitive function was analyzed at 1, 10, and 180 days after surgery. Craniotomy mouse model, cerebrospinal fluid of patients with the appearance of IL-6 storm after craniotomy, and IL-6 at the same concentration stimulation model were established. Behavioral tests, fluorescence in situ hybridization (FISH), pathological means, western blot, and ELISA (enzyme-linked immune-sorbent assay) were performed for verification. CSF from patients after craniotomy caused disturbance of consciousness in mice, affected neuronal damage in the hypothalamus, activation of microglia in the hypothalamus, and decreased expression of barrier proteins in the hypothalamus and brain. The large amount of interleukin-6 in CSF after craniotomy was found to be mainly derived from astrocytes. The IL-6 level in CSF after craniotomy correlated inversely with patients’ performance in MoCA test. High levels of IL-6 in the cerebrospinal fluid derived from astrocytes after craniotomy may lead to disruption of the brain-cerebrospinal fluid barrier, most notably around the hypothalamus, which might result in inflammatory activation of microglia to damage the hypothalamic neurons and impaired cognitive function/more gradual cognitive repairment in patients after craniotomy with the appearance of IL-6 storm.





Similar content being viewed by others
Data Availability
The data and material are available.
References
Li Y, Yang S, Zhou X, Lai R, Tan D (2022) A retrospective cohort study of neuroendoscopic surgery versus traditional craniotomy on surgical success rate, postoperative complications, and prognosis in patients with acute intracerebral hemorrhage. Comput Intell Neurosci 2022:2650795
Zhou D, Wei D, ** and interventional embolization on treatment efficacy, cognitive function and recovery of patients complicated with subarachnoid hemorrhage. Am J Transl Res 13(5):5117–5126
Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Ranganath C, Redish AD (2017) Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci 20(11):1434–1447
Evered LA, Chan MTV, Han R, Chu MHM, Cheng BP, Scott DA et al (2021) Anaesthetic depth and delirium after major surgery: a randomised clinical trial. Br J Anaesth 127(5):704–712
Hirano T, Taga T, Nakano N, Yasukawa K, Kashiwamura S, Shimizu K, et al (1985) Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc Natl Acad Sci U S A 82(16):5490-5494
Rothaug M, Becker-Pauly C, Rose-John S (2016) The role of interleukin-6 signaling in nervous tissue. Biochim Biophys Acta 1863(6 Pt A):1218–27
Bulzacka E, Boyer L, Schürhoff F, Godin O, Berna F, Brunel L et al (2016) Chronic peripheral inflammation is associated with cognitive impairment in schizophrenia: results from the multicentric FACE-SZ dataset. Schizophr Bull 42(5):1290–1302
Benveniste EN (1998) Cytokine actions in the central nervous system. Cytokine Growth Factor Rev 9(3–4):259–275
Lilja A, Nordborg C, Brun A, Salford LG, Aman P (2001) Expression of the IL-6 family cytokines in human brain tumors. Int J Oncol 19(3):495–499
Frei K, Fredrikson S, Fontana A, Link H (1991) Interleukin-6 is elevated in plasma in multiple sclerosis. J Neuroimmunol 31(2):147–153
Maimone D, Guazzi GC, Annunziata P (1997) IL-6 detection in multiple sclerosis brain. J Neurol Sci 146(1):59–65
Ali C, Nicole O, Docagne F, Lesne S, MacKenzie ET, Nouvelot A et al (2000) Ischemia-induced interleukin-6 as a potential endogenous neuroprotective cytokine against NMDA receptor-mediated excitotoxicity in the brain. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 20(6):956–966
Jadhav V, Solaroglu I, Obenaus A, Zhang JH (2007) Neuroprotection against surgically induced brain injury. Surg Neurol 67(1):15–20 (discussion)
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I et al (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699
Borland E, Nägga K, Nilsson PM, Minthon L, Nilsson ED, Palmqvist S (2017) The Montreal cognitive assessment: normative data from a large Swedish population-based cohort. J Alzheimer’s Dis: JAD 59(3):893–901
Chucair-Elliott AJ, Conrady C, Zheng M, Kroll CM, Lane TE, Carr DJ (2014) Microglia-induced IL-6 protects against neuronal loss following HSV-1 infection of neural progenitor cells. Glia 62(9):1418–1434
Hirota H, Kiyama H, Kishimoto T, Taga T (1996) Accelerated nerve regeneration in mice by upregulated expression of interleukin (IL) 6 and IL-6 receptor after trauma. J Exp Med 183(6):2627–2634
Yang P, Wen H, Ou S, Cui J, Fan D (2012) IL-6 promotes regeneration and functional recovery after cortical spinal tract injury by reactivating intrinsic growth program of neurons and enhancing synapse formation. Exp Neurol 236(1):19–27
Patanella AK, Zinno M, Quaranta D, Nociti V, Frisullo G, Gainotti G et al (2010) Correlations between peripheral blood mononuclear cell production of BDNF, TNF-alpha, IL-6, IL-10 and cognitive performances in multiple sclerosis patients. J Neurosci Res 88(5):1106–1112
Schuitemaker A, Dik MG, Veerhuis R, Scheltens P, Schoonenboom NS, Hack CE et al (2009) Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging 30(11):1885–1889
Tan EK, Chan LL (2007) Neurovascular compression syndromes and hypertension: clinical relevance. Nat Clin Pract Neurol 3(8):416–417
Perry VH (2004) The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun 18(5):407–413
Teeling JL, Perry VH (2009) Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience 158(3):1062–1073
van Gool WA, van de Beek D, Eikelenboom P (2010) Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 375(9716):773–775
Gross PM, Weindl A (1987) Peering through the windows of the brain. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 7(6):663–672
Palkovits M (1984) Neuropeptides in the hypothalamo-hypophyseal system: lateral retrochiasmatic area as a common gate for neuronal fibers towards the median eminence. Peptides 5(Suppl 1):35–39
Desai TR, Leeper NJ, Hynes KL, Gewertz BL (2002) Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J Surg Res 104(2):118–123
Cohen SS, Min M, Cummings EE, Chen X, Sadowska GB, Sharma S et al (2013) Effects of interleukin-6 on the expression of tight junction proteins in isolated cerebral microvessels from yearling and adult sheep. NeuroImmunoModulation 20(5):264–273
Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, Quick KL et al (2009) IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One 4(5):e5518
Rosenblat JD, Brietzke E, Mansur RB, Maruschak NA, Lee Y, McIntyre RS (2015) Inflammation as a neurobiological substrate of cognitive impairment in bipolar disorder: evidence, pathophysiology and treatment implications. J Affect Disord 188:149–159
Willis EF, MacDonald KPA, Nguyen QH, Garrido AL, Gillespie ER, Harley SBR et al (2020) Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell 180(5):833–46.e16
Monsour M, Croci DM, Agazzi S (2022) The role of IL-6 in TBI and PTSD, a potential therapeutic target? Clin Neurol Neurosurg 218:107280
Li Z, **ao J, Xu X, Li W, Zhong R, Qi L, Chen J, Cui G, Wang S, Zheng Y, Qiu Y, Li S, Zhou X, Lu Y, Lyu J, Zhou B, Zhou J, **g N, Wei B, Hu J, Wang H (2021) M-CSF, IL-6, and TGF-β promote generation of a new subset of tissue repair macrophage for traumatic brain injury recovery. Sci Adv 7(11):eabb6260
Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD et al (2018) Parkin and PINK1 mitigate STING-induced inflammation. Nature 561(7722):258–262
Li N, Wang JX, Huo TT, Zhao JR, Wang TJ (2021) Associations of IL-1β and IL-6 gene polymorphisms with Parkinson’s disease. Eur Rev Med Pharmacol Sci 25(2):890–897
Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS et al (2018) IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep 8(1):12050
Fujihara K, Bennett JL, de Seze J, Haramura M, Kleiter I, Weinshenker BG, Kang D, Mughal T, Yamamura T (2020) Interleukin-6 in neuromyelitis optica spectrum disorder pathophysiology. Neurol Neuroimmunol Neuroinflamm. 7(5):e841
Hirano T (2021) IL-6 in inflammation, autoimmunity and cancer. Int Immunol 33(3):127–148
Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B (2009) How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost 102(2):215–222
Williams G, Harrold JA, Cutler DJ (2020) The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box. Proc Nutr Soc 59(3):385-96
Saper CB, Scammell TE, Lu J (2005) Hypothalamic regulation of sleep and circadian rhythms. Nature 437(7063):1257–1263
Miller AA, Spencer SJ (2014) Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun 42:10–21
Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M et al (2016) Major depressive disorder. Nat Rev Dis Primers 2:16065
Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S (1998) Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 57(1):1–9
Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y (2001) Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke 32(5):1208–1215
López-Gambero AJ, Martínez F, Salazar K, Cifuentes M, Nualart F (2019) Brain glucose-sensing mechanism and energy homeostasis. Mol Neurobiol 56(2):769–796
Santos Fontanez SE, De Jesus O (2023) Neurohypophysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–
Jiang H, Gallet S, Klemm P, Scholl P, Folz-Donahue K, Altmüller J et al (2020) MCH neurons regulate permeability of the median eminence barrier. Neuron 107(2):306–19.e9
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N et al (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161(3):653–660
Geng J, Wang L, Zhang L, Qin C, Song Y, Ma Y et al (2018) Blood-brain barrier disruption induced cognitive impairment is associated with increase of inflammatory cytokine. Front Aging Neurosc 10:129
Rochfort KD, Collins LE, McLoughlin A, Cummins PM (2016) Tumour necrosis factor-α-mediated disruption of cerebrovascular endothelial barrier integrity in vitro involves the production of proinflammatory interleukin-6. J Neurochem 136(3):564–572
Rom S, Heldt NA, Gajghate S, Seliga A, Reichenbach NL, Persidsky Y (2020) Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci Rep 10(1):7274
Rochfort KD, Collins LE, Murphy RP, Cummins PM (2014) Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: consequences for interendothelial adherens and tight junctions. PLoS One 9(7):e101815
Camire RB, Beaulac HJ, Willis CL (2015) Transitory loss of glia and the subsequent modulation in inflammatory cytokines/chemokines regulate paracellular claudin-5 expression in endothelial cells. J Neuroimmunol 284:57–66
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201
Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Muñoz Maniega S et al (2009) Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol 65(2):194–202
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and dysfunction of the blood-brain barrier. Cell 163(5):1064–1078
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12(12):723–738
Wardlaw JM, Sandercock PA, Dennis MS, Starr J (2003) Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 34(3):806–812
Lightman SL, Birnie MT, Conway-Campbell BL (2020) Dynamics of ACTH and Cortisol Secretion and Implications for Disease. Endocr Rev 41(3):bnaa002
Tan BL, Norhaizan ME (2019) Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients 11(11):2579
Acknowledgements
Deepest gratitude goes first and foremost to those who provided their constant encouragement and guidance. Without their consistent and illuminating instruction, this thesis could not have reached its present form. Besides, heartfelt gratitude is given to those who led me into the world of translation. I am also greatly indebted to all the professors and teachers who have instructed and helped me a lot in the period of the research. I am indebted to the participants of this study who generously volunteered their time and effort. Without their participation, this research would not have been possible. Last but not least, I would like to express my deepest gratitude to my family and friends for their unwavering support, understanding, and encouragement throughout this journey. Their love, patience, and belief in me have been the driving force behind my perseverance. In conclusion, I extend my appreciation to all individuals who have played a part, big or small, in the completion of this research. Their contributions have been indispensable and greatly appreciated.
Funding
Province natural science fund of Guangdong (2021A1515111147), Medical Scientific Research Foundation of Guangdong Province of China (B2018245), Clinical Research Program of Nanfang Hospital, Southern Medical University (2018CR029), Clinical Research Startup Program of Southern Medical University by High-level University Construction Funding of Guangdong Provincial Department of Education (LC2016PY012, LC2019ZD004), and President Foundation of Nanfang Hospital, Southern Medical University (2018C017).
Author information
Authors and Affiliations
Contributions
Y. B., S. Q., F. M., and B. Q. contributed to the conception of the study; H. H., A. Z., K. Z., C. Y., and X. L. performed the experiment; H. H., F. M., F. L., H. M., and W. D. contributed significantly to the analysis and manuscript preparation; H. H. and Y. B. performed the data analyses and wrote the manuscript; Y. B. and B. Q. helped perform the analysis with constructive discussions.
Corresponding authors
Ethics declarations
Ethics Approval
The study was approved by the medical ethics committee of Nanfang Hospital, Southern Medical University (no. NFEC-2023–414). And the animal study was approved by the Committee on the Ethics of Animal Experiments of Nanfang Hospital, Southern Medical University (no. NFYY-2021–0826). The medical ethics committee approved a waiver of consent for collection of these data as part of routine clinical care and quality control.
Consent to Participate
All the patients were consent to participate in the research.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haorun Huang, **xian Liao, An Zhang, and Binghui Qiu are co-first authors of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
12035_2023_3693_MOESM1_ESM.tif
Supplementary Fig. 1. Co-staining fluorescence pattern of IL-6 mRNA probe and GFAP antibody in the craniotomy area (Red fluorescence was IL-6 mRNA probe. Green fluorescence was GFAP antibody expression. The blue-purple fluorescence was DAPI. MERGE is the mixed image) (TIF 1344 KB)
12035_2023_3693_MOESM2_ESM.tif
Supplementary Fig. 2. Co-staining fluorescence pattern of IL-6 mRNA probe and GFAP antibody in the craniotomy area (magnification 10 times and 20 times. Red fluorescence was IL-6 mRNA probe. Green fluorescence was GFAP antibody expression. The blue-purple fluorescence was DAPI. MERGE is the mixed image) (TIF 1028 KB)
12035_2023_3693_MOESM5_ESM.tif
Supplementary Fig. 5. Co-staining fluorescence pattern of IL-6 mRNA probe and GFAP antibody in the craniotomy area after using IL-6 Receptor Antagonist (Tocilizumab) (TIF 869 KB)
12035_2023_3693_MOESM6_ESM.tif
Supplementary Fig. 6. Multivariate correlation analysis on the clinical data of the patients. The results showed that the patient's cerebrospinal fluid IL-6 value was an independent influencing factor for MoCA (p=0.038) (TIF 169 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, H., Liao, X., Zhang, A. et al. Cerebrospinal Fluid from Patients After Craniotomy with the Appearance of Interleukin-6 Storm Can Activate Microglia to Damage the Hypothalamic Neurons in Mice. Mol Neurobiol 61, 2707–2718 (2024). https://doi.org/10.1007/s12035-023-03693-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03693-1