Abstract
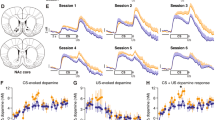
The activity of the midbrain dopamine system reflects the valence of environmental events and modulates various brain structures to modify an organism’s behavior. A series of recent studies reported that the direct and indirect pathways in the striatum are critical for instrumental learning, but the dynamic changes in dopamine neuron activity that occur during negative reinforcement learning are still largely unclear. In the present study, by using a negative reinforcement learning paradigm employing foot shocks as aversive stimuli, bidirectional changes in substantia nigra pars compacta (SNc) dopamine neuron activity in the learning and habituation phases were observed. The results showed that in the learning phase, before mice had mastered the skill of esca** foot shocks, the presence of foot shocks induced a transient reduction in the activity of SNc dopamine neurons; however, in the habituation phase, in which the learned skill was automated, it induced a transient increase. Microinjection of a dopamine D1 receptor (D1R) or D2 receptor (D2R) antagonist into the dorsomedial striatum (DMS) significantly impaired learning behavior, suggesting that the modulatory effects of dopamine on both the direct and indirect pathways are required. Moreover, during the learning phase, excitatory synaptic transmission to DMS D2R-expressing medium spiny neurons (D2-MSNs) was potentiated. However, upon completion of the learning and habituation phases, the synapses onto D1R-expressing medium spiny neurons (D1-MSNs) were potentiated, and those onto D2-MSNs were restored to normal levels. The bidirectional changes in both SNc dopamine neuron activity and DMS synaptic plasticity might be the critical neural correlates for negative reinforcement learning.






Similar content being viewed by others
Data Availability
All data generated during this study are available from the corresponding author on request.
Code Availability
Not applicable.
Change history
21 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12035-021-02573-w
Abbreviations
- SNc:
-
Substantia nigra pars compacta
- DA:
-
Dopamine
- DMS:
-
Dorsomedial striatum
- MSNs:
-
Medium spiny neurons
- D1R:
-
Dopamine D1 receptor
- D2R:
-
Dopamine D2 receptor
- ERF:
-
The event-related fluorescence
- sEPSCs:
-
Spontaneous excitatory postsynaptic currents
References
Nakanishi S, Hikida T, Yawata S (2014) Distinct dopaminergic control of the direct and indirect pathways in reward-based and avoidance learning behaviors. Neurosci 282:49–59. https://doi.org/10.1016/j.neuroscience.2014.04.026
Graybiel AM (2008) Habits, rituals, and the evaluative brain. Ann Rev Neurosci 31:359–387. https://doi.org/10.1146/annurev.neuro.29.051605.112851
Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Ann Rev Neurosci 34:441–466. https://doi.org/10.1146/annurev-neuro-061010-113641
Bromberg-Martin ES, Matsumoto M, Hikosaka O (2010) Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68:815–834. https://doi.org/10.1016/j.neuron.2010.11.022
**ao X, Deng H, Furlan A, Yang T, Zhang X, Hwang GR, Tucciarone J, Wu P et al (2020) A genetically defined compartmentalized striatal direct pathway for negative reinforcement. Cell 183:211–227. https://doi.org/10.1016/j.cell.2020.08.032
Costa RM (2007) Plastic corticostriatal circuits for action learning: what’s dopamine got to do with it? Ann N Y Acad Sci 1104:172–191. https://doi.org/10.1196/annals.1390.015
Yin HH, Mulcare SP, Hilário MRF, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM et al (2009) Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci 12:333–341. https://doi.org/10.1038/nn.2261
Kravitz AV, Kreitzer AC (2012) Striatal mechanisms underlying movement, reinforcement, and punishment. Physiol 27(3):1–20. https://doi.org/10.1152/physiol.00004.2012
Cui G, Jun SB, ** X, Pham MD, Vogel SS, Lovinger DM, Costa RM (2013) Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494(7436):238–242. https://doi.org/10.1038/nature11846
Tecuapetla F, ** X, Lima SQ, Costa RM (2016) Complementary contributions of striatal projection pathways to action initiation and execution. Cell 166:703–715. https://doi.org/10.1016/j.cell.2016.06.032
Shan Q, Ge M, Christie MJ, Balleine BW (2014) The acquisition of goal-directed actions generates opposing plasticity in direct and indirect pathways in dorsomedial striatum. J Neurosci 34:9196–9201. https://doi.org/10.1523/JNEUROSCI.0313-14.2014
Fiorillo CD, Yun SR, Song MR (2013) Diversity and homogeneity in responses of midbrain dopamine neurons. J Neurosci 33(11):4693–4709. https://doi.org/10.1523/JNEUROSCI.3886-12.2013
Suri RE, Schultz W (1998) Learning of sequential movements by neural network model with dopamine-like reinforcement signal. Exp Brain Res 121:350–354. https://doi.org/10.1007/s002210050467
Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Sci 275:1593–1599. https://doi.org/10.1126/science.275.5306.1593
Zhang X, Kim J, Tonegawa S (2020) Amygdala reward neurons form and store fear extinction memory. Neuron 105:1077–1093. https://doi.org/10.1016/j.neuron.2019.12.025
Yu T, Li Y, Hu Q, Wang F, Yuan S, Li C, Li JP, Cui JL et al (2020) Ketamine contributes to the alteration of Ca2+ transient evoked by behavioral tests in the prelimbic area of mPFC: a study on chronic CORT-induced depressive mice. Neurosci Lett 735:135220. https://doi.org/10.1016/j.neulet.2020.135220
Nasehi M, Hasanvand S, Khakpai F, Zarrindast MR (2019) The effect of CA1 dopaminergic system on amnesia induced by harmane in mice. Acta Neurol Belg 119:369–377. https://doi.org/10.1007/s13760-018-0926-8
Piri M, Ayazi E, Zarrindast MR (2013) Involvement of the dorsal hippocampal dopamine D2 receptors in histamine-induced anxiogenic-like effects in mice. Neurosci Lett 550:139–144. https://doi.org/10.1016/j.neulet.2013.07.009
Yao L, Li Y, Qian Z, Wu M, Yang H, Chen N (2019) Loss of control over mild aversive events produces significant helplessness in mice. Behav Brain Res 376:112173. https://doi.org/10.1016/j.bbr.2019.112173
Wang XY, Qiao YH, Dai ZH, Sui N, Shen F, Zhang JJ, Liang J (2019) Medium spiny neurons of the anterior dorsomedial striatum mediate reversal learning in a cell-type-dependent manner. Brain Struct Funct 224:419–434. https://doi.org/10.1007/s00429-018-1780-4
Huang S, Uusisaari MY (2013) Physiologycal temperature during brain slicing enhances the quality of acute slice preparations. Front Cellular Neurosci 7:48. https://doi.org/10.3389/fncel.2013.00048
Cadwell CR, Palasantza A, Jiang X, Berens P, Deng Q, Yilmaz M, Reimer J, Shenet S et al (2016) Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat Biotechnol 34:199–203. https://doi.org/10.1038/nbt.3445
Zhong WX, Li Y, Feng QR, Luo MM (2017) Learning and stress shape the reward response patterns of serotonin neurons. J Neurosci 37:8863–8875. https://doi.org/10.1523/JNEUROSCI.1181-17.2017
Wang DQ, Li Y, Feng QR, Guo QC, Zhou JF, Luo MM (2017) Learning shapes the aversion and reward responses of lateral habenula neurons. Elife 6:1–20. https://doi.org/10.7554/elife.23045
Daubner SC, Le T, Wang S (2011) Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 508:1–12. https://doi.org/10.1016/j.abb.2010.12.017
Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ et al (2014) Natural neural projection dynamics underlying social behavior. Cell 157:1535–1551. https://doi.org/10.1016/j.cell.2014.05.017
Gallistel CR, Fairhurst SF, Balsam P (2004) The learning curve: implications of a quantitative analysis. PNAS 101:13124–13131. https://doi.org/10.1073/pnas.0404965101
Choi WY, Balsam PD, Horvitz JC (2005) Extended habit training reduces dopamine mediation of appetitive response expression. J Neurosci 25:6729–6733. https://doi.org/10.1523/JNEUROSCI.1498-05.2005
Costa RM, Cohen D, Nicolelis MAL (2004) Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol 14:1124–1134. https://doi.org/10.1016/j.cub.2004.06.053
André VM, Cepeda C, Cummings DM, Jocoy EL, Fisher YE, Yang XW, Levine MS (2010) Dopamine modulation of excitatory currents in the striatum is dictated by the expression of D1 or D2 receptor and modified by endocannabinoids. Eur J Neurosci 31:14–28. https://doi.org/10.1111/j.1460-9568.2009.07047.x
Shen W, Flajolet M, Greengard P, Surmeier DJ (2008) Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321:848–851. https://doi.org/10.1126/science.1160575
Ade KK, Wan Y, Chen M, Gloss B, Calakos N (2011) An improved BAC transgenic fluorescent reporter line for sensitive and specific identification of striatonigral medium spiny neurons. Front Syst Neurosci 5:1–9. https://doi.org/10.3389/fnsys.2011.00032
Heintz N (2002) BAC to the future: the use of BAC transgenic mice for neuroscience research. Nat Rev Neurosci 2:861–870. https://doi.org/10.1038/35104049
Cepeda C, AndréVM YI, Wu N, Kleiman-Weiner M, Levine MS (2008) Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur J Neurosci 27:671–682. https://doi.org/10.1111/j.1460-9568.2008.06038.x
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27. https://doi.org/10.1152/jn.1998.80.1.1
Higley MJ, Sabatini BL (2010) Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci 13:958–966. https://doi.org/10.1038/nn.2592
Tritsch NX, Sabatini BL (2012) Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76:33–50. https://doi.org/10.1016/j.neuron.2012.09.023
Hikosaka O, Takikawa Y, Kawagoe R (2000) Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80:953–978. https://doi.org/10.1152/physrev.2000.80.3.953
Mink JW (2003) The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol 60:1365–1368. https://doi.org/10.1001/archneur.60.10.1365
Tepper JM, Wilson CJ, Koós T (2008) Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res Rev 58:272–281. https://doi.org/10.1016/j.brainresrev.2007.10.008
Tecuapetla F, Koo´s T, Tepper JM, Kabbani N, Yeckel MF (2009) Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. J Neurosci 29:8977–8990. https://doi.org/10.1523/JNEUROSCI.6145-08.2009
André VM, Cepeda C, Cummings DM, Jocoy EL, Fisher YE, Yang XW, Levine MS (2010) Dopamine modulation of excitatory currents in the striatum is dictated by the expression of D1 or D2 receptors and modified by endocannabinoids. Eur J Neurosci 31:14–28. https://doi.org/10.1111/j.1460-9568.2009.07047.x
** X, Costa RM (2015) Sha** action sequences in basal ganglia circuits. Curr Opin Neurobiol 33:188–196. https://doi.org/10.1016/j.conb.2015.06.011
Geddes CE, Li H, ** X (2018) Optogenetic Editing Reveals the Hierarchical Organization of Learned Action Sequences. Cell 174:32–43. https://doi.org/10.1016/j.cell.2018.06.012
Acknowledgements
We thank Dr. Zhiqiang Liu of Shaanxi Normal University for expertise with electrophysiological recording.
Funding
This research was supported by the National Natural Science Foundation of China (Project No. 81971285; 11727813; 82071516), and by the Fundamental Research Funds for the Central Universities (Project No. GK202005001, GK202105001), Shaanxi Normal University.
Author information
Authors and Affiliations
Contributions
Conceptualization: WR. Methodology: ZJD, LY, MLW, and YYD. Validation: WR, YFT, ZJD, LY, and YX-L. Formal analysis: WR, YFT, and ZJD. Investigation: ZJD, ZQQ, QQC, and CLW. Resources: YX-L, WR, and YFT. Writing, original draft: WR and ZJD. Writing, review and editing: WR, YFT, ZJD, LY, MLW, YYD, and QQC. Supervision: WR and YFT. Funding acquisition: WR and YFT. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All the experimental procedures were approved by the Medicine Animal Care and Use Committee of Shaanxi Normal University conformed to the Guide for the National Institutional Animal Care and the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals and their suffering.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diao, Z., Yao, L., Cheng, Q. et al. Involvement of Midbrain Dopamine Neuron Activity in Negative Reinforcement Learning in Mice. Mol Neurobiol 58, 5667–5681 (2021). https://doi.org/10.1007/s12035-021-02515-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02515-6