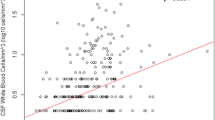
Abstract
HIV-associated neurocognitive disorder (HAND) remains prevalent despite antiretroviral therapy and involves white matter damage in the brain. Although iron is essential for myelination and myelin maintenance/repair, its role in HAND is largely unexplored. We tested the hypotheses that cerebrospinal fluid (CSF) heavy-chain ferritin (Fth1) and transferrin, proteins integral to iron delivery and myelination, are associated with neurocognitive performance in people with HIV (PWH). Fth1, transferrin, and the pro-inflammatory cytokines TNF-α and IL-6 were quantified in CSF at baseline (entry) in 403 PWH from a prospective observational study who underwent serial, comprehensive neurocognitive assessments. Associations of Fth1 and transferrin with Global Deficit Score (GDS)-defined neurocognitive performance at baseline and 30–42 months of follow-up were evaluated by multivariable regression. While not associated with neurocognitive performance at baseline, higher baseline CSF Fth1 predicted significantly better neurocognitive performance over 30 months in all PWH (p < 0.05), in PWH aged < 50 at 30, 36, and 42 months (all p < 0.05), and in virally suppressed PWH at all three visit time-points (all p < 0.01). Higher CSF transferrin was associated with superior neurocognitive performance at all visits, primarily in viremic individuals (all p < 0.05). All associations persisted after adjustment for neuro-inflammation. In summary, higher CSF Fth1 is neuroprotective over prolonged follow-up in all and virally suppressed PWH, while higher CSF transferrin may be most neuroprotective during viremia. We speculate that higher CSF levels of these critical iron-delivery proteins support improved myelination and consequently, neurocognitive performance in PWH, providing a rationale for investigating their role in interventions to prevent and/or treat HAND.



Similar content being viewed by others
Data Availability
All data and datasets used for the analyses reported herein will be made available upon request.
References
Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A et al (2016) HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol 12(4):234–248. https://doi.org/10.1038/nrneurol.2016.27
Alakkas A, Ellis RJ, Watson CW, Umlauf A, Heaton RK, Letendre S, Collier A, Marra C et al (2019) White matter damage, neuroinflammation, and neuronal integrity in HAND. J Neuro-Oncol 25(1):32–41. https://doi.org/10.1007/s13365-018-0682-9
Fennema-Notestine C, Thornton-Wells TA, Hulgan T, Letendre S, Ellis RJ, Franklin DR Jr, Anderson AM, Heaton RK et al (2019) Iron-regulatory genes are associated with neuroimaging measures in HIV infection. Brain Imaging Behav 14:2037–2049. https://doi.org/10.1007/s11682-019-00153-0
Kallianpur AR, Wang Q, Jia P, Hulgan T, Zhao Z, Letendre SL, Ellis RJ, Heaton RK et al (2016) Anemia and Red blood cell indices predict HIV-associated neurocognitive impairment in the highly active antiretroviral therapy era. J Infect Dis 213(7):1065–1073. https://doi.org/10.1093/infdis/jiv754
Velichkovska M, Surnar B, Nair M, Dhar S, Toborek M (2019) Targeted mitochondrial COQ10 delivery attenuates antiretroviral-drug-induced senescence of neural progenitor cells. Mol Pharm 16(2):724–736. https://doi.org/10.1021/acs.molpharmaceut.8b01014
Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F, Gannon PJ, Mankowski J et al (2014) Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neuro-Oncol 20(1):39–53. https://doi.org/10.1007/s13365-013-0227-1
Borsa M, Ferreira PL, Petry A, Ferreira LG, Camargo MM, Bou-Habib DC, Pinto AR (2015) HIV infection and antiretroviral therapy lead to unfolded protein response activation. Virol J 12:77. https://doi.org/10.1186/s12985-015-0298-0
Festa L, Roth LM, B KJ, Geiger JD, Jordan-Sciutto KL, Grinspan JB (2019) Protease inhibitors, saquinavir and darunavir, inhibit oligodendrocyte maturation: implications for lysosomal stress. J NeuroImmune Pharmacol 16:169–180. https://doi.org/10.1007/s11481-019-09893-8
Fields JA, Ellis RJ (2019) HIV in the cART era and the mitochondrial: immune interface in the CNS. Int Rev Neurobiol 145:29–65. https://doi.org/10.1016/bs.irn.2019.04.003
Wendelken LA, Jahanshad N, Rosen HJ, Busovaca E, Allen I, Coppola G, Adams C, Rankin KP et al (2016) ApoE epsilon4 is associated with cognition, brain integrity, and atrophy in HIV over age 60. J Acquir Immune Defic Syndr 73(4):426–432. https://doi.org/10.1097/QAI.0000000000001091
Geffin R, McCarthy M (2018) Aging and apolipoprotein E in HIV infection. J Neuro-Oncol 24(5):529–548. https://doi.org/10.1007/s13365-018-0660-2
Patton SM, Wang Q, Hulgan T, Connor JR, Jia P, Zhao Z, Letendre SL, Ellis RJ et al (2017) Cerebrospinal fluid (CSF) biomarkers of iron status are associated with CSF viral load, antiretroviral therapy, and demographic factors in HIV-infected adults. Fluids Barriers CNS 14(1):11. https://doi.org/10.1186/s12987-017-0058-1
Chiou B, Neely EB, McDevitt DS, Simpson IA, Connor JR (2020) Transferrin and H-ferritin involvement in brain iron acquisition during postnatal development: impact of sex and genotype. J Neurochem 152(3):381–396. https://doi.org/10.1111/jnc.14834
Kuhn S, Gritti L, Crooks D, Dombrowski Y (2019) Oligodendrocytes in development, myelin generation and beyond. Cells 8(11). https://doi.org/10.3390/cells8111424
Belaidi AA, Bush AI (2016) Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem 139(Suppl 1):179–197. https://doi.org/10.1111/jnc.13425
Chiou B, Lucassen E, Sather M, Kallianpur A, Connor J (2018) Semaphorin4A and H-ferritin utilize Tim-1 on human oligodendrocytes: a novel neuro-immune axis. Glia 66(7):1317–1330. https://doi.org/10.1002/glia.23313
Fennema-Notestine C, Ellis RJ, Archibald SL, Jernigan TL, Letendre SL, Notestine RJ, Taylor MJ, Theilmann RJ et al (2013) Increases in brain white matter abnormalities and subcortical gray matter are linked to CD4 recovery in HIV infection. J Neuro-Oncol 19(4):393–401. https://doi.org/10.1007/s13365-013-0185-7
Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T et al (2011) Clinical factors related to brain structure in HIV: the CHARTER study. J Neuro-Oncol 17(3):248–257. https://doi.org/10.1007/s13365-011-0032-7
Arosio P, Carmona F, Gozzelino R, Maccarinelli F, Poli M (2015) The importance of eukaryotic ferritins in iron handling and cytoprotection. Biochem J 472(1):1–15. https://doi.org/10.1042/BJ20150787
Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, Sougrat R, Morgenstern A et al (2010) Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 116(9):1574–1584. https://doi.org/10.1182/blood-2009-11-253815
Todorich B, Zhang X, Connor JR (2011) H-ferritin is the major source of iron for oligodendrocytes. Glia 59(6):927–935. https://doi.org/10.1002/glia.21164
Chiou B, Connor JR (2018) Emerging and dynamic biomedical uses of ferritin. Pharmaceuticals (Basel) 11(4). https://doi.org/10.3390/ph11040124
Franco PG, Pasquini LA, Perez MJ, Rosato-Siri MV, Silvestroff L, Pasquini JM (2015) Paving the way for adequate myelination: the contribution of galectin-3, transferrin and iron. FEBS Lett 589(22):3388–3395. https://doi.org/10.1016/j.febslet.2015.08.001
Leitner DF, Connor JR (2012) Functional roles of transferrin in the brain. Biochim Biophys Acta 1820(3):393–402. https://doi.org/10.1016/j.bbagen.2011.10.016
Rosato-Siri MV, Marziali LN, Mattera V, Correale J, Pasquini JM (2021) Combination therapy of apo-transferrin and thyroid hormones enhances remyelination. Glia 69(1):151–164. https://doi.org/10.1002/glia.23891
Ganz T, Nemeth E (2015) Iron homeostasis in host defence and inflammation. Nat Rev Immunol 15(8):500–510. https://doi.org/10.1038/nri3863
Rouault TA (2013) Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci 14(8):551–564. https://doi.org/10.1038/nrn3453
Larsen B, Bourque J, Moore TM, Adebimpe A, Calkins ME, Elliott MA, Gur RC, Gur RE et al (2020) Longitudinal development of brain iron is linked to cognition in youth. J Neurosci 40(9):1810–1818. https://doi.org/10.1523/JNEUROSCI.2434-19.2020
Kallianpur AR, Gittleman H, Letendre S, Ellis R, Barnholtz-Sloan JS, Bush WS, Heaton R, Samuels DC et al (2019) Cerebrospinal fluid ceruloplasmin, haptoglobin, and vascular endothelial growth factor are associated with neurocognitive impairment in adults with HIV infection. Mol Neurobiol 56(5):3808–3818. https://doi.org/10.1007/s12035-018-1329-9
Mehta SR, Perez-Santiago J, Hulgan T, Day TR, Barnholtz-Sloan J, Gittleman H, Letendre S, Ellis R et al (2017) Cerebrospinal fluid cell-free mitochondrial DNA is associated with HIV replication, iron transport, and mild HIV-associated neurocognitive impairment. J Neuroinflammation 14(1):72. https://doi.org/10.1186/s12974-017-0848-z
Deisenhammer F, Miller RF, Brink NS, Harrison MJ, Thompson EJ (1997) Cerebrospinal fluid ferritin in HIV infected patients with acute neurological episodes. Genitourin Med 73(3):181–183
Perrella O, Finelli L, Munno I, Perrella A, Soscia E, Carrieri PB (1993) Cerebrospinal fluid ferritin in human immunodeficiency virus infection: a marker of neurologic involvement? J Infect Dis 168(4):1079–1080
Heaton RK, Franklin DR Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP et al (2015) Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 60(3):473–480. https://doi.org/10.1093/cid/ciu862
Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL et al (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75(23):2087–2096. https://doi.org/10.1212/WNL.0b013e318200d727
Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, Gelman BB, McArthur JC et al (2012) Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol 26(6):894–908. https://doi.org/10.1080/13854046.2012.694479
Butts CA, Swift J, Kang SG, Di Costanzo L, Christianson DW, Saven JG, Dmochowski IJ (2008) Directing noble metal ion chemistry within a designed ferritin protein. Biochemistry 47(48):12729–12739. https://doi.org/10.1021/bi8016735
Sacri AS, Ferreira D, Khoshnood B, Gouya L, Barros H, Chalumeau M (2017) Stability of serum ferritin measured by immunoturbidimetric assay after storage at -80 degrees C for several years. PLoS One 12(12):e0188332. https://doi.org/10.1371/journal.pone.0188332
Spencer BR, Brodsky JP, Holley GC, Foster GA, Winton C, Stramer SL (2019) Expanded feasibility of ferritin testing: stability of ferritin stored as whole blood and validation of plastic tubes. Transfusion 59(11):3424–3430. https://doi.org/10.1111/trf.15513
Jansen EH, Beekhof PK, Schenk E (2013) Long-term stability of biomarkers of the iron status in human serum and plasma. Biomarkers 18(4):365–368. https://doi.org/10.3109/1354750X.2013.781223
Haverkamp N, Beauducel A (2017) Violation of the Sphericity assumption and its effect on type-I error rates in repeated measures ANOVA and multi-level linear models (MLM). Front Psychol 8:1841. https://doi.org/10.3389/fpsyg.2017.01841
Ganz T (2019) The discovery of the iron-regulatory hormone hepcidin. Clin Chem 65(10):1330–1331. https://doi.org/10.1373/clinchem.2019.306407
Sheppard DP, Iudicello JE, Bondi MW, Doyle KL, Morgan EE, Massman PJ, Gilbert PE, Woods SP (2015) Elevated rates of mild cognitive impairment in HIV disease. J Neuro-Oncol 21(5):576–584. https://doi.org/10.1007/s13365-015-0366-7
Wei J, Hou J, Su B, Jiang T, Guo C, Wang W, Zhang Y, Chang B et al (2020) The prevalence of Frascati-Criteria-Based HIV-Associated Neurocognitive Disorder (HAND) in HIV-infected adults: a systematic review and meta-analysis. Front Neurol 11:581346. https://doi.org/10.3389/fneur.2020.581346
Jensen BK, Roth LM, Grinspan JB, Jordan-Sciutto KL (2019) White matter loss and oligodendrocyte dysfunction in HIV: a consequence of the infection, the antiretroviral therapy or both? Brain Res 1724:146397. https://doi.org/10.1016/j.brainres.2019.146397
Hoare J, Fouche JP, Phillips N, Joska JA, Paul R, Donald KA, Thomas KG, Stein DJ (2015) White matter micro-structural changes in ART-naive and ART-treated children and adolescents infected with HIV in South Africa. AIDS 29(14):1793–1801. https://doi.org/10.1097/QAD.0000000000000766
Li RL, Sun J, Tang ZC, Zhang JJ, Li HJ (2018) Axonal chronic injury in treatment-naive HIV+ adults with asymptomatic neurocognitive impairment and its relationship with clinical variables and cognitive status. BMC Neurol 18(1):66. https://doi.org/10.1186/s12883-018-1069-5
Liu H, Xu E, Liu J, **ong H (2016) Oligodendrocyte Injury and Pathogenesis of HIV-1-Associated Neurocognitive Disorders. Brain Sci 6(3). https://doi.org/10.3390/brainsci6030023
Zou S, Fuss B, Fitting S, Hahn YK, Hauser KF, Knapp PE (2015) Oligodendrocytes are targets of HIV-1 Tat: NMDA and AMPA receptor-mediated effects on survival and development. J Neurosci 35(32):11384–11398. https://doi.org/10.1523/JNEUROSCI.4740-14.2015
Chang HC, Bayeva M, Taiwo B, Palella FJ Jr, Hope TJ, Ardehali H (2015) Short communication: high cellular iron levels are associated with increased HIV infection and replication. AIDS Res Hum Retrovir 31(3):305–312. https://doi.org/10.1089/aid.2014.0169
Armitage AE, Stacey AR, Giannoulatou E, Marshall E, Sturges P, Chatha K, Smith NM, Huang X et al (2014) Distinct patterns of hepcidin and iron regulation during HIV-1, HBV, and HCV infections. Proc Natl Acad Sci U S A 111(33):12187–12192. https://doi.org/10.1073/pnas.1402351111
Drakesmith H, Chen N, Ledermann H, Screaton G, Townsend A, Xu XN (2005) HIV-1 Nef down-regulates the hemochromatosis protein HFE, manipulating cellular iron homeostasis. Proc Natl Acad Sci U S A 102(31):11017–11022. https://doi.org/10.1073/pnas.0504823102
Clifford DB (2017) HIV-associated neurocognitive disorder. Curr Opin Infect Dis 30(1):117–122. https://doi.org/10.1097/QCO.0000000000000328
Ances BM, Letendre SL (2019) CROI 2019: neurologic complications of HIV disease. Top Antivir Med 27(1):26–33
Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR (2009) Oligodendrocytes and myelination: the role of iron. Glia 57(5):467–478. https://doi.org/10.1002/glia.20784
Alejandro D. Roth MTN (2016) Oligodendrocytes: functioning in a delicate balance between high metabolic requirements and oxidative damage. In: Glial Cells in Health and Disease of the CNS, vol 949. Springer, Cham, pp 167-181. doi:https://doi.org/10.1007/978-3-319-40764-7_8
McCarthy RC, Sosa JC, Gardeck AM, Baez AS, Lee CH, Wessling-Resnick M (2018) Inflammation-induced iron transport and metabolism by brain microglia. J Biol Chem 293(20):7853–7863. https://doi.org/10.1074/jbc.RA118.001949
Han J, Seaman WE, Di X, Wang W, Willingham M, Torti FM, Torti SV (2011) Iron uptake mediated by binding of H-ferritin to the TIM-2 receptor in mouse cells. PLoS One 6(8):e23800. https://doi.org/10.1371/journal.pone.0023800
Zhang X, Surguladze N, Slagle-Webb B, Cozzi A, Connor JR (2006) Cellular iron status influences the functional relationship between microglia and oligodendrocytes. Glia 54(8):795–804. https://doi.org/10.1002/glia.20416
Hulet SW, Heyliger SO, Powers S, Connor JR (2000) Oligodendrocyte progenitor cells internalize ferritin via clathrin-dependent receptor mediated endocytosis. J Neurosci Res 61(1):52–60. https://doi.org/10.1002/1097-4547(20000701)61:1<52::AID-JNR6>3.0.CO;2-T
Li L, Fang CJ, Ryan JC, Niemi EC, Lebron JA, Bjorkman PJ, Arase H, Torti FM et al (2010) Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci U S A 107(8):3505–3510. https://doi.org/10.1073/pnas.0913192107
Wan R, Cheli VT, Santiago-Gonzalez DA, Rosenblum SL, Wan Q, Paez PM (2020) Impaired postnatal myelination in a conditional knockout mouse for the ferritin heavy chain in oligodendroglial cells. J Neurosci 40(40):7609–7624. https://doi.org/10.1523/JNEUROSCI.1281-20.2020
Cheng HT, Yen CJ, Chang CC, Huang KT, Chen KH, Zhang RY, Lee PY, Miaw SC et al (2015) Ferritin heavy chain mediates the protective effect of heme oxygenase-1 against oxidative stress. Biochim Biophys Acta 1850(12):2506–2517. https://doi.org/10.1016/j.bbagen.2015.09.018
Mukherjee C, Kling T, Russo B, Miebach K, Kess E, Schifferer M, Pedro LD, Weikert U et al (2020) Oligodendrocytes provide antioxidant defense function for neurons by secreting ferritin heavy chain. Cell Metab 32(2):259–272 e210. https://doi.org/10.1016/j.cmet.2020.05.019
Chiou B, Neely E, Kallianpur A, Connor JR (2019) Semaphorin4A causes loss of mature oligodendrocytes and demyelination in vivo. J Neuroinflammation 16(1):28. https://doi.org/10.1186/s12974-019-1420-9
Leitner DF, Todorich B, Zhang X, Connor JR (2015) Semaphorin4A is cytotoxic to oligodendrocytes and is elevated in microglia and multiple sclerosis. ASN Neuro 7(3):175909141558750. https://doi.org/10.1177/1759091415587502
Thompson K, Menzies S, Muckenthaler M, Torti FM, Wood T, Torti SV, Hentze MW, Beard J et al (2003) Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J Neurosci Res 71(1):46–63. https://doi.org/10.1002/jnr.10463
Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY (1999) Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal 13(6):273–279. https://doi.org/10.1002/(sici)1098-2825(1999)13:6<273::aid-jcla4>3.0.co;2-x
Daruich A, Le Rouzic Q, Jonet L, Naud MC, Kowalczuk L, Pournaras JA, Boatright JH, Thomas A, Turck N, Moulin A, Behar-Cohen F, Picard E (2019) Iron is neurotoxic in retinal detachment and transferrin confers neuroprotection. Sci Adv 5 (1):eaau9940. doi:https://doi.org/10.1126/sciadv.aau9940
Rouault TA, Zhang DL, Jeong SY (2009) Brain iron homeostasis, the choroid plexus, and localization of iron transport proteins. Metab Brain Dis 24(4):673–684. https://doi.org/10.1007/s11011-009-9169-y
Acknowledgements
We are grateful to all of the individuals who participated in this study. We also acknowledge the efforts of other CHARTER Study Group members who assisted in recruitment at participating study sites:
Other CHARTER Study Group members
In addition to the authors, the CHARTER study is affiliated with the following six participating study sites: Johns Hopkins University; the Icahn School of Medicine at Mount Sinai; University of California, San Diego; University of Texas, Galveston; University of Washington, Seattle; and Washington University, St. Louis. CHARTER is headquartered at the University of California, San Diego and includes Director: Igor Grant, M.D.; Co-Directors: Scott L. Letendre, M.D., Ronald J. Ellis, M.D., Ph.D., Thomas D. Marcotte, Ph.D.; Center Manager: Donald Franklin, Jr.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D.; Laboratory and Virology Component: Scott Letendre, M.D. (Co-P.I.), Davey M. Smith, M.D. (Co-P.I.).; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Matthew Dawson; Imaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Michael J Taylor, Ph.D., Rebecca Theilmann, Ph.D.; Data Management Component: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Component: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D.; Johns Hopkins University Site: Ned Sacktor (P.I.), Vincent Rogalski; Icahn School of Medicine at Mount Sinai Site: Susan Morgello, M.D. (Co-P.I.) and David Simpson, M.D. (Co-P.I.), Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.); University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Sher Storey, PA-C.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., Mengesha Teshome, M.D.
Code Availability
Not applicable.
Funding
This work was supported by National Institutes of Health grant 1R01 MH095621 (to AK and TH). The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) Study was also supported by National Institutes of Health awards NIH R01 MH107345 (to SL and RH).
Author information
Authors and Affiliations
Contributions
The first and last authors contributed equally to this work. All other authors reviewed the manuscript, provided helpful comments, and agreed to its submission for publication.
Corresponding author
Ethics declarations
Ethics Approval
CHARTER is a multi-center study which has received continuous approval by the institutional review boards (IRBs) of all participating institutions/sites. IRB approval numbers can be provided upon request.
Consent to Participate
All CHARTER study participants provided written informed consent for the parent study that provided the de-identified CSF samples and data for this analysis.
Consent for Publication
All authors provided consent for publication.
Conflict of Interest
Authors Kaur, Bush, Letendre, Franklin, Ellis, Hulgan, Heaton, Samuels, and Kallianpur have no potential conflicts of interest to disclose. Author S. Patton is a paid consultant for SideroBiosciences, Inc., and J.R. Connor, a longstanding collaborator, is co-founder and Chairman of the Board of SideroBiosciences, which has a product in clinical trials for treating iron deficiency.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplemental Fig 1:
Changes in neurocognitive performance in CHARTER study participants, as measured by the Global Deficit Score (GDS) over time, in all three tertiles at baseline of CSF Fth1 and transferrin (panels a and b, respectively; both p<0.05 for tertiles 3 (red) vs. 1 (blue) of Fth1 over at 30 and 42 months (adjusting for comorbidity and plasma HIV RNA), and all p<0.01 for tertiles 3 vs. 1 of CSF transferrin at 30, 36, and 42 months, adjusting for comorbidity, zidovudine use and plasma HIV RNA). (PNG 183 kb)
Supplemental Fig 2:
Changes in GDS over time, in all three tertiles at CSF Fth1 among individuals with no/minimal comorbidity (panel a, p<0.05 at 30, 36, and 42 months) adjusting for plasma HIV RNA and in individuals who were virally suppressed (panel b, p<0.001 at 30, 36, and 42 months of follow-up), adjusting for comorbidity. (PNG 181 kb)
Supplemental Fig 3:
Changes in GDS over time in tertiles 3 vs. 1 of CSF transferrin among virally suppressed individuals (p>0.05 at 30, 36, and 42 months) adjusting for comorbidity and zidovudine use. (PNG 88 kb)
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Kaur, H., Bush, W.S., Letendre, S.L. et al. Higher CSF Ferritin Heavy-Chain (Fth1) and Transferrin Predict Better Neurocognitive Performance in People with HIV. Mol Neurobiol 58, 4842–4855 (2021). https://doi.org/10.1007/s12035-021-02433-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02433-7