Abstract
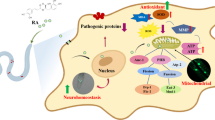
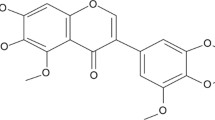
Parkinsonism is an age-associated neurodegenerative disorder characterized by aggregation of α-synuclein (α-syn) protein in the substantia nigra region, degeneration of dopaminergic neurons, and deregulated lipid metabolism. Currently, only symptomatic relief has been provided by FDA-approved therapeutic approaches for Parkinson’s disease (PD). The present study aims to evaluate the potential of wedelolactone (WDL), a natural occurring coumestan found in Eclipta alba to mitigate the parkinsonism in Caenorhabditis elegans disease model. In the present studies, supplementation with 37.5 μM WDL exhibited a reduction in the level of α-syn in an age-dependent manner (22% at day 5, p < 0.05; and 16% at day 10, p < 0.001, n = 30), along with improvement in neuronal health through basal movement, and elevated the dopamine levels evident through 1-nonanol repulsion results in wild-type and diseased worms. Moreover, WDL augmented the mitochondrial health in wild-type, PD-diseased, and mev-1 mutant worms that establish the inherent activity of WDL in the alleviation of oxidative stress. Furthermore, WDL supplementation significantly decreases the neutral lipid and triglyceride level and also alleviates protein carbonyl level in PD disease condition. The overall investigation will provide a pioneer to the future insights of PD research related to plant-based drugs. qPCR studies after WDL supplementation revealed alteration of genes involved in the regulation of various stress-responsive (sod-5, gst-4, skn-1), α-syn-suppressing (lrk-1, ymel-1, lagr-1, grk-1), and mitochondrial (pink-1) genes. All together, these findings support that the WDL is a promising candidate to combat age-related multi-factorial PD pathology associated with protein misfolding and accumulation. The results provide sufficient information in the development of therapeutic medicines from natural products for improving the health.





Similar content being viewed by others
References
Caiazza MC, Lang C, Wade-Martins R (2020) What we can learn from iPSC-derived cellular models of Parkinson’s disease. Prog Brain Res 252:3
Johansen KK, Torp SH, Farrer MJ, Gustavsson EK, Aasly JO (2018) A case of Parkinson’s disease with no Lewy body pathology due to a homozygous exon deletion in Parkin. Case Rep Neurol Med 2018:1–4
Monzani E, Nicolis S, Dell'Acqua S, Capucciati A, Bacchella C, Zucca FA, Mosharov EV, Sulzer D et al (2019) Dopamine, oxidative stress and protein–quinone modifications in Parkinson’s and other neurodegenerative diseases. Angew Chem Int Ed 58(20):6512–6527
Jackson M, Naidoo K, Birch-Machin M (2019) Oxidative stress, metabolism and photoaging—the role of mitochondria. Cutaneous Photoaging 19:105
Elmazoglu Z, Kovacikova L, Stefek M, Karasu C (2019) Novel carboxymethylated mercaptotriazinoindole derivatives as potential inhibitors of aldo-keto reductase (akr1b1) ameliorate hyperglycemia mediated and 6-ohda-induced neurotoxicity in pc12 cells: in vitro hyperglycemic Parkinson’s disease model. ispbs–5 proceedings book:47
Wei J, Du M, Bai Y (2019) Correlations of melatonin and glutathione levels with oxidative stress mechanism in Parkinson’s disease. Zhongguo yi xue ke xue yuan xue bao Acta Acad Med Sin 41(2):183–187
Shin J-h, Jo A, Lee Y, Dawson TM, Dawson VL (2019) Methods of preventing or treating Parkinson’s disease by the farnesylation of paris. Google Patents
Vida C, Kobayashi H, Garrido A, Martínez de Toda I, Carro E, Molina JA, De la Fuente M (2019) Lymphoproliferation impairment and oxidative stress in blood cells from early Parkinson’s disease patients. Int J Mol Sci 20(3):771
Ullah R, Khan M, Shah SA, Saeed K, Kim MO (2019) Natural antioxidant anthocyanins—a hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients 11(6):1195
Smita SS, Raj Sammi S, Laxman TS, Bhatta RS, Pandey R (2017) Shatavarin IV elicits lifespan extension and alleviates parkinsonism in Caenorhabditis elegans. Free Radic Res 51(11–12):954–969
Farooqui AA, Farooqui T (2019) Therapeutic potentials of curcumin in Parkinson’s disease. In: Curcumin for neurological and psychiatric disorders. Elsevier, pp 333–344
Paradkar P, Mishra L, Joshi J, Dandekar S, Vaidya R, Vaidya A (2017) In vitro macrophage activation: a technique for screening anti-inflammatory, immunomodulatory and anticancer activity of phytomolecules
Chakraborty SP (2019) Medicinal plants and cervical cancer therapy: an overview. J Pharmacogn Phytochem 8(3):3633–3641
Badmanaban R, Padathil MS, Majeed S, Joy DM, Parveen H (2019) A review on plant derived multitarget therapeutic phytomolecule-embelin. World J Curr Med Pharm Res 166–173
Shankar A, Gopinath S, Shareef I (2020) Phytobioactives from Alstonia scholaris—an elixir against cancer. Our Heritage 68(30):4989–5007
Zhao H, Cheng S, Zhang L, Dong H, Zhang Y, Wang X (2019) Ultra-high-pressure-assisted extraction of wedelolactone and isodemethylwedelolactone from Ecliptae Herba and purification by high-speed counter-current chromatography. Biomed Chromatogr 33(6):e4497
Yang J, Tao L, Liu B, You X, Zhang C, **e H, Li R (2019) Wedelolactone attenuates pulmonary fibrosis partly through AMPK activation and regulating Raf-MAPKs signaling pathway. Front Pharmacol 10:151
He J-B, Chen M-H, Lin D-K (2017) New insights into the tonifying kidney-yin herbs and formulas for the treatment of osteoporosis. Arch Osteoporos 12(1):14
Sarveswaran S, Ghosh R, Parikh R, Ghosh J (2016) Wedelolactone, an anti-inflammatory botanical, interrupts c-Myc oncogenic signaling and synergizes with enzalutamide to induce apoptosis in prostate cancer cells. Mol Cancer Ther 15(11):2791–2801
Pratap GMS, Manoj KM, Sai SA, Sujatha B, Sreedevi E (2012) Evaluation of three medicinal plants for anti-microbial activity. Ayu 33(3):423–428
Kučírková T, Stiborek M, Dúcka M, Navrátilová J, Pristov JB, Popović-Bijelić A, Vojvodić S, Preisler J et al (2018) Anti-cancer effects of wedelolactone: interactions with copper and subcellular localization. Metallomics 10(10):1524–1531
Das S, Mukherjee P, Chatterjee R, Jamal Z, Chatterji U (2019) Enhancing chemosensitivity of breast cancer stem cells by downregulating SOX2 and ABCG2 using wedelolactone-encapsulated nanoparticles. Mol Cancer Ther 18(3):680–692
Zhang S, Kuhn JR (2018) Cell isolation and culture. In: WormBook: the online review of C. elegans biology [Internet]. WormBook
Rathor L, Pant A, Nagar A, Tandon S, Trivedi S, Pandey R (2017) Trachyspermum ammi L.(Carom) oil induces alterations in SOD-3, GST-4 expression and prolongs lifespan in Caenorhabditis elegans. Proc Natl Acad Sci India B Biol Sci 87(4):1355–1362
Pandey T, Sammi SR, Nooreen Z, Mishra A, Ahmad A, Bhatta RS, Pandey R (2019) Anti-ageing and anti-Parkinsonian effects of natural flavonol, tambulin from Zanthoxyllum aramatum promotes longevity in Caenorhabditis elegans. Exp Gerontol 120:50–61
Maulik M, Mitra S, Bult-Ito A, Taylor BE, Vayndorf EM (2017) Behavioral phenoty** and pathological indicators of Parkinson’s disease in C. elegans models. Front Genet 8:77
Tayo LL, Lin Y-H, Lin S-L, Gou Y-Y, Hsu Y-C, Hou W-C, Huang K-L, Chao H-R (2019) Fine particulate matter-induced toxic effects in an animal model of caenorhabditis elegans. Aerosol Air Qual Res 19:1068–1078
Bose A, Beal MF (2019) Mitochondrial dysfunction and oxidative stress in induced pluripotent stem cell models of Parkinson’s disease. Eur J Neurosci 49(4):525–532
Hallett PJ, Engelender S, Isacson O (2019) Lipid and immune abnormalities causing age-dependent neurodegeneration and Parkinson’s disease. J Neuroinflammation 16(1):153
Chalorak P, Jattujan P, Nobsathian S, Poomtong T, Sobhon P, Meemon K (2018) Holothuria scabra extracts exhibit anti-Parkinson potential in C. elegans: a model for anti-Parkinson testing. Nutr Neurosci 21(6):427–438
Paul F, Teixeira-Castro A, Costa M, Lindsay V, Fiúza-Fernandes J, Goua M, Bermano G, Russell W et al (2019) GST-4-dependent suppression of neurodegeneration in C. elegans models of Parkinson’s and Machado-Joseph disease by rapeseed pomace extract supplementation. Front Neurosci 13:1091
Chiaradia E, Renzone G, Scaloni A, Caputo M, Costanzi E, Gambelunghe A, Muzi G, Avellini L et al (2019) Protein carbonylation in dopaminergic cells exposed to rotenone. Toxicol Lett 309:20–32
Hernandez LF, Obeso I, Costa RM, Redgrave P, Obeso JA (2019) Dopaminergic vulnerability in Parkinson disease: the cost of humans’ habitual performance. Trends Neurosci 42:375–383
Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR (2016) Oxidative stress and Parkinson’s. Parkinson’s disease: cell vulnerability and disease progression
Romuk EB, Szczurek W, Oleś M, Gabrysiak A, Skowron M, Nowak P, Birkner E (2017) The evaluation of the changes in enzymatic antioxidant reserves and lipid peroxidation in chosen parts of the brain in an animal model of Parkinson disease. Adv Clin Exp Med 26(6):953–959
Bose A, Beal MF (2016) Mitochondrial dysfunction in Parkinson’s disease. J Neurochem 139:216–231
Reed TT, Butterfield DA (2017) Protein carbonylation in brains of subjects with selected neurodegenerative disorders. Protein Carbonylation: Principles, Analysis, and Biological Implications 167–205
Sharma VD, Lyons KE, Pahwa R (2018) Amantadine extended-release capsules for levodopa-induced dyskinesia in patients with Parkinson’s disease. Ther Clin Risk Manag 14:665–673
Addo MG, Cossard R, Pichard D, Obiri-Danso K, Rötig A, Delahodde A (2016) Identification of new genes involved in mtDNA maintenance in Caenorhabditis elegans that could represent candidate genes for mitochondrial diseases. Int J Curr Microbiol App Sci 5(6):179–189
Rocha EM, De Miranda B, Sanders LH (2018) Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol Dis 109:249–257
Butler B, Sambo D, Khoshbouei H (2017) Alpha-synuclein modulates dopamine neurotransmission. J Chem Neuroanat 83:41–49
Sammi SR, Agim ZS, Cannon JR (2018) From the cover: Harmane-induced selective dopaminergic neurotoxicity in Caenorhabditis elegans. Toxicol Sci 161(2):335–348
Walker B, Grewal AS, Grayson NK, Harris LR, Diokpa C, Aceves T (2018) Induced mutation in Caenorhabditis elegans causes dopamine resistance.
Martinez BA, Caldwell KA, Caldwell GA (2017) C. elegans as a model system to accelerate discovery for Parkinson disease. Curr Opin Genet Dev 44:102–109
Van Ham TJ, Thijssen KL, Breitling R, Hofstra RM, Plasterk RH, Nollen EA (2008) C. elegans model identifies genetic modifiers of α-synuclein inclusion formation during aging. PLoS Genet 4(3)
Islam MT (2017) Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 39(1):73–82
Shefa U, Jeong NY, Song IO, Chung H-J, Kim D, Jung J, Huh Y (2019) Mitophagy links oxidative stress conditions and neurodegenerative diseases. Neural Regen Res 14(5):749
Ludtmann MH, Abramov AY (2018) Mitochondrial calcium imbalance in Parkinson’s disease. Neurosci Lett 663:86–90
Paul R, Choudhury A, Kumar S, Giri A, Sandhir R, Borah A (2017) Cholesterol contributes to dopamine-neuronal loss in MPTP mouse model of Parkinson’s disease: involvement of mitochondrial dysfunctions and oxidative stress. PLoS One 12(2)
Mantegazza AR, Marks MS (2016) Pink light on mitochondria in autoimmunity and Parkinson disease. Cell Metab 24(1):11–12
Liu J, Liu W, Li R, Yang H (2019) Mitophagy in Parkinson’s disease: from pathogenesis to treatment. Cells 8(7):712
Sies H, Berndt C, Jones DP (2017) Oxidative stress. Annu Rev Biochem 86:715–748
Shamoto-Nagai M, Hisaka S, Naoi M, Maruyama W (2018) Modification of α-synuclein by lipid peroxidation products derived from polyunsaturated fatty acids promotes toxic oligomerization: its relevance to Parkinson disease. J Clin Biochem Nutr 62(3):207–212
Killinger BA, Melki R, Brundin P, Kordower JH (2019) Endogenous alpha-synuclein monomers, oligomers and resulting pathology: let’s talk about the lipids in the room. NPJ Parkinson’s Dis 5(1):1–8
Sokolowska E, Błachnio-Zabielska AU (2019) The role of ceramides in insulin resistance. Front Endocrinol 10:577
Goswami DG, Kant R, Ammar DA, Agarwal C, Gomez J, Agarwal R, Saba LM, Fritz KS et al (2020) Toxic consequences and oxidative protein carbonylation from chloropicrin exposure in human corneal epithelial cells. Toxicol Lett 322:1–11
Haus JM, Thyfault JP (2018) Therapeutic potential of carbonyl-scavenging carnosine derivative in metabolic disorders. J Clin Invest 128(12):5198–5200
Acknowledgments
The authors are highly grateful to the Caenorhabditis Genetics Centre (CGC), Minneapolis, MN, USA, which is funded by the NIH, National Centre for Research Resources (USA), for providing the C. elegans strains. The authors are grateful to the Director, CSIR–Central Institute of Medicinal and Aromatic Plants, Lucknow, India, for his valuable support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, S., Trivedi, S., Pandey, T. et al. Wedelolactone Mitigates Parkinsonism Via Alleviating Oxidative Stress and Mitochondrial Dysfunction Through NRF2/SKN-1. Mol Neurobiol 58, 65–77 (2021). https://doi.org/10.1007/s12035-020-02080-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02080-4