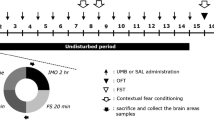
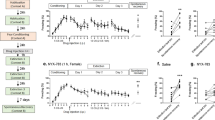
Abstract
We previously reported that fatty acid-binding protein 3 (FABP3) knockout (Fabp3 −/−) mice exhibit abnormal dopamine-related behaviors such as enhanced dopamine D2 receptor antagonist-induced catalepsy behaviors. Here, we report that Fabp3 null mice exhibit cognitive deficits, hyperlocomotion and impaired fear extinction, and thus show post-traumatic stress disorder (PTSD)-like behaviors. Notably, chronic administration of ramelteon (1.0 mg/kg, p.o.), a melatonin receptor agonist, improved all PTSD-like behaviors tested in Fabp3 −/− mice. Relevant to mechanisms underlying impaired fear extinction, we observed significantly reduced levels of Ca2+/calmodulin-dependent protein kinase II (CaMKII) autophosphorylation without changes in ERK phosphorylation in the anterior cingulate cortex (ACC). Inversely, CaMKII autophosphorylation increased in the basolateral amygdala (BLA) but remained relatively unchanged in hippocampus of Fabp3 −/− mice. Likewise, the number of c-Fos-positive neurons in BLA significantly increased after exposure to contextual fear conditions but remained unchanged in the central nucleus of the amygdala (CeA). Importantly, chronic ramelteon administration (1.0 mg/kg, p.o.) restored abnormal c-Fos expression and CaMKII autophosphorylation in the ACC and BLA of Fabp3 −/− mice. Finally, the melatonin receptor antagonist luzindole (2.5 mg/kg, i.p.) blocked ramelteon-dependent improvements. Taken together, Fabp3 −/− mice show PTSD-like behaviors, and ramelteon is a likely attractive candidate for PTSD therapy.







Similar content being viewed by others
Abbreviations
- ACC:
-
Anterior cingulate cortex
- ANOVA:
-
Analysis of variance
- BLA:
-
Basolateral nucleus of the amygdala
- CaMKII:
-
Ca2+/calmodulin-dependent protein kinase II
- CeA:
-
Central nucleus of the amygdala
- CMC:
-
Carboxymethylcellulose
- DAB:
-
3′3′-Diaminobenzidine-tetrahydrochloride
- D2LR:
-
Dopamine D2 receptor long isoform
- D2R:
-
Dopamine D2 receptor
- ERK:
-
Extracellular signal-regulated kinase
- Fabp3 −/− :
-
FABP3 knockout
- Fabp7 −/− :
-
FABP7 knockout
- FABP3:
-
Fatty acid-binding protein 3
- FABPs:
-
Fatty acid-binding proteins
- fMRI:
-
Functional magnetic resonance imaging
- GABA:
-
γ-Aminobutyric acid
- LCPUFAs:
-
Long-chain polyunsaturated fatty acids
- MRI:
-
Magnetic resonance imaging
- NMDA:
-
N-methyl-d-aspartate
- PBS:
-
Phosphate-buffered saline
- PTSD:
-
Post-traumatic stress disorder
- SEM:
-
Standard error of the mean
- WT:
-
Wild-type
References
Gordon N (1997) Nutrition and cognitive function. Brain and Development 19(3):165–170
Sakayori N, Kikkawa T, Tokuda H, Kiryu E, Yoshizaki K, Kawashima H, Yamada T, Arai H et al (2015) Maternal dietary imbalance between omega-6 and omega-3 polyunsaturated fatty acids impairs neocortical development via epoxy metabolites. Stem Cells. In press
Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP (2003) Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr Res 62(3):195–204
Vancassel S, Durand G, Barthélémy C, Lejeune B, Martineau J, Guilloteau D, Andrès C, Chalon S (2001) Plasma fatty acid levels in autistic children. Prostaglandins Leukot Essent Fatty Acids 65(1):1–7
Maekawa M, Takashima N, Matsumata M, Ikegami S, Kontani M, Hara Y, Kawashima H, Owada Y et al (2009) Arachidonic acid drives postnatal neurogenesis and elicits a beneficial effect on prepulse inhibition, a biological trait of psychiatric illnesses. PLoS One 4(4):e5085
Mellor JE, Laugharne JD, Peet M (1995) Schizophrenic symptoms and dietary intake of n-3 fatty acids. Schizophr Res 118(1):85–86
Kotani S, Sakaguchi E, Warashina S, Matsukawa N, Ishikura Y, Kiso Y, Sakakibara M, Yoshimoto T et al (2006) Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res 56(2):159–164
Horrocks LA, Yeo YK (1999) Health benefits of docosahexaenoic acid (DHA). Pharmacol Res 40(3):211–225
Coe NR, Bernlohr DA (1998) Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim Biophys Acta 11391(3):287–306
Liu RZ, Li X, Godbout R (2008) A novel fatty acid-binding protein (FABP) gene resulting from tandem gene duplication in mammals: transcription in rat retina and testis. Genomics 92(6):436–445
Furuhashi M, Hotamisligil GS (2008) Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7(6):489–503
Owada Y, Yoshimoto T, Kondo H (1996) Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in develo** and mature rat brains. J Chem Neuroanat 12(2):113–122
Owada Y (2008) Fatty acid binding protein: localization and functional significance in the brain. Tohoku J Exp Med 214(3):213–220
Matsumata M, Sakayori N, Maekawa M, Owada Y, Yoshikawa T, Osumi N (2012) The effects of Fabp7 and Fabp5 on postnatal hippocampal neurogenesis in the mouse. Stem Cells 30(7):1532–1543
Owada Y, Abdelwahab SA, Kitanaka N, Sakagami H, Takano H, Sugitani Y, Sugawara M, Kawashima H et al (2006) Altered emotional behavioral responses in mice lacking brain-type fatty acid-binding protein gene. Eur J Neurosci 24(1):175–187
Shimamoto C, Ohnishi T, Maekawa M, Watanabe A, Ohba H, Arai R, Iwayama Y, Hisano Y et al (2014) Functional characterization of FABP3, 5 and 7 gene variants identified in schizophrenia and autism spectrum disorder and mouse behavioral studies. Hum Mol Genet 23(24):6495–6511
Shioda N, Yamamoto Y, Watanabe M, Binas B, Owada Y, Fukunaga K (2010) Heart-type fatty acid binding protein regulates dopamine D2 receptor function in mouse brain. J Neurosci 30(8):3146–3155
Takeuchi Y, Fukunaga K (2003) Differential subcellular localization of two dopamine D2 receptor isoforms in transfected NG108-15 cells. J Neurochem 85(4):1064–1074
Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62(6):617–627
Fullerton CS, Ursano RJ, Wang L (2004) Acute stress disorder, posttraumatic stress disorder, and depression in disaster or rescue workers. Am J Psychiatry 161(8):1370–1376
Ressler KJ, Mayberg HS (2007) Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10(9):1116–1124
Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G et al (1993) Deficits in short-term memory in posttraumatic stress disorder. Am J Psychiatry 150(7):1015–1019
Yehuda R, Keefe RS, Harvey PD, Levengood RA, Gerber DK, Geni J, Siever LJ (1995) Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry 152(1):137–139
Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN Jr, Sutker PB (2002) Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology 16(1):5–14
Moser DA, Aue T, Suardi F, Kutlikova H, Cordero MI, Rossignol AS, Favez N, Rusconi Serpa S et al (2015) Violence-related PTSD and neural activation when seeing emotionally charged male-female interactions. Soc Cogn Affect Neurosci 10(5):645–653
Yu B, Cui SY, Zhang XQ, Cui XY, Li SJ, Sheng ZF, Cao Q, Huang YL et al (2015) Different neural circuitry is involved in physiological and psychological stress-induced PTSD-like “nightmares” in rats. Sci Rep 5:15976
Matsuoka Y, Nishi D, Nakaya N, Sone T, Hamazaki K, Hamazaki T, Koido Y (2011) Attenuating posttraumatic distress with omega-3 polyunsaturated fatty acids among disaster medical assistance team members after the Great East Japan Earthquake: the APOP randomized controlled trial. BMC Psychiatry 11:132
Matsuoka Y (2011) Clearance of fear memory from the hippocampus through neurogenesis by omega-3 fatty acids: a novel preventive strategy for posttraumatic stress disorder? Biopsychosoc Med 5:3
Matsuoka Y, Nishi D, Yonemoto N, Hamazaki K, Hashimoto K, Hamazaki T (2010) Omega-3 fatty acids for secondary prevention of posttraumatic stress disorder after accidental injury: an open-label pilot study. J Clin Psychopharmacol 30(2):217–219
Stehle JH, Saade A, Rawashdeh O, Ackermann K, Jilg A, Sebestény T, Maronde E (2011) A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J Pineal Res 51(1):17–43
Comai S, Gobbi G (2014) Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology. J Psychiatry Neurosci 39(1):6–21
Doyen C, Mighiu D, Kaye K, Colineaux C, Beaumanoir C, Mouraeff Y, Rieu C, Paubel P et al (2011) Melatonin in children with autistic spectrum disorders: recent and practical data. Eur Child Adolesc Psychiatry 20(5):231–239
Huang F, Yang Z, Liu X, Li CQ (2014) Melatonin facilitates extinction, but not acquisition or expression, of conditional cued fear in rats. BMC Neurosci 15:86
Schaap FG, Binas B, Danneberg H, van der Vusse GJ, Glatz JF (1999) Impaired long-chain fatty acid utilization by cardiac myocytes isolated from mice lacking the heart-type fatty acid binding protein gene. Circ Res 85(4):329–337
Yabuki Y, Fukunaga K (2013) Oral administration of glutathione improves memory deficits following transient brain ischemia by reducing brain oxidative stress. Neuroscience 250:394–407
Yabuki Y, Ohizumi Y, Yokosuka A, Mimaki Y, Fukunaga K (2014) Nobiletin treatment improves motor and cognitive deficits seen in MPTP-induced Parkinson model mice. Neuroscience 259:126–141
Miyamoto Y, Fukuda T (2015) Immunohistochemical study on the neuronal diversity and three-dimensional organization of the mouse entopeduncular nucleus. Neurosci Res 94:37–49
Fukunaga K, Goto S, Miyamoto E (1988) Immunohistochemical localization of Ca2+/calmodulin-dependent protein kinase II in rat brain and various tissues. J Neurochem 51:1070–1078
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates. Academic Press, San Diego
Yamamoto Y, Sharifi K, Islam A, Ebrahimi M, Kagawa Y, Yasumoto Y, Miyazaki H, Kawamura S et al (2013) Fatty acid binding protein 3 (FABP3) regulates cognitive function and an anxiety behavior. Neuroscience 2013 Abstract 701.08
Maren S, Quirk GJ (2004) Neuronal signaling of fear memory. Nat Rev Neurosci 5(11):844–852
Lanuza E, Moncho-Bogani J, Ledoux JE (2008) Unconditioned stimulus pathways to the amygdala: effects of lesions of the posterior intralaminar thalamus on foot-shock-induced c-Fos expression in the subdivisions of the lateral amygdala. Neuroscience 155(3):959–968
Lüthi A, Lüscher C (2014) Pathological circuit function underlying addiction and anxiety disorders. Nat Neurosci 17(12):1635–1643
Amaral OB, Roesler R (2008) Targeting the NMDA receptor for fear-related disorders. Recent Pat CNS Drug Discov 3(3):166–178
Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ (2007) Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron 53(6):871–880
Lerea LS, Butler LS, McNamara JO (1992) NMDA and non-NMDA receptor-mediated increase of c-fos mRNA in dentate gyrus neurons involves calcium influx via different routes. J Neurosci 12(8):2973–2981
Collingridge GL, Isaac JT, Wang YT (2004) Receptor trafficking and synaptic plasticity. Nat Rev Neurosci 5(12):952–962
Miyamoto E (2006) Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci 100(5):433–442
O’Doherty DC, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J (2015) A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res 232(1):1–33
Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A (2000) Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci 911:369–391
Myers KM, Davis M (2007) Mechanisms of fear extinction. Mol Psychiatry 12(2):120–150
Etkin A, Wager TD (2007) Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164(10):1476–1488
Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD (2005) Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord 88(1):79–86
Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC et al (2001) An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry 50(12):932–942
Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R et al (2003) Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A 100(15):9039–9043
Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK (2000) Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 47(9):769–776
Hoerndli FJ, Wang R, Mellem JE, Kallarackal A, Brockie PJ, Thacker C, Madsen DM, Maricq AV (2015) Neuronal activity and CaMKII regulate kinesin-mediated transport of synaptic AMPARs. Neuron 86(2):457–474
Akirav I, Raizel H, Maroun M (2006) Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci 23(3):758–764
Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS (2004) Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci 24(15):3810–3815
Mátyás F, Lee J, Shin HS, Acsády L (2014) The fear circuit of the mouse forebrain: connections between the mediodorsal thalamus, frontal cortices and basolateral amygdala. Eur J Neurosci 39(11):1810–1823
Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP et al (2010) Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci 13(4):482–488
Feng P, Feng T, Chen Z, Lei X (2014) Memory consolidation of fear conditioning: bi-stable amygdala connectivity with dorsal anterior cingulate and medial prefrontal cortex. Soc Cogn Affect Neurosci 9(11):1730–1737
Weaver DR, Stehle JH, Stopa EG, Reppert SM (1993) Melatonin receptors in human hypothalamus and pituitary: implications for circadian and reproductive responses to melatonin. J Clin Endocrinol Metab 76(2):295–301
Lacoste B, Angeloni D, Dominguez-Lopez S, Calderoni S, Mauro A, Fraschini F, Descarries L, Gobbi G (2015) Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J Pineal Res 58(4):397–417
Domínguez-Alonso A, Valdés-Tovar M, Solís-Chagoyán H, Benítez-King G (2015) Melatonin stimulates dendrite formation and complexity in the hilar zone of the rat hippocampus: participation of the Ca++/Calmodulin complex. Int J Mol Sci 16(1):1907–1927
Tian Y, Yabuki Y, Moriguchi S, Fukunaga K, Mao PJ, Hong LJ, Lu YM, Wang R et al (2014) Melatonin reverses the decreases in hippocampal protein serine/threonine kinases observed in an animal model of autism. J Pineal Res 56(1):1–11
Zeng W, Mak DO, Li Q, Shin DM, Foskett JK, Muallem S (2003) A new mode of Ca2+ signaling by G protein-coupled receptors: gating of IP3 receptor Ca2+ release channels by Gbetagamma. Curr Biol 13(10):872–876
Nishiyama K, Shintani Y, Hirai K, Yoshikubo S (2009) Molecular cloning and pharmacological characterization of monkey MT1 and MT2 melatonin receptors showing high affinity for the agonist ramelteon. J Pharmacol Exp Ther 330(3):855–863
Abraham AD, Neve KA, Lattal KM (2016) Activation of D1/5 dopamine receptors: a common mechanism for enhancing extinction of fear and reward-seeking behaviors. Neuropsychopharmacology
El-Ghundi M, O’Dowd BF, George SR (2007) Insights into the role of dopamine receptor systems in learning and memory. Rev Neurosci 18(1):37–66
Mueller D, Bravo-Rivera C, Quirk GJ (2010) Infralimbic D2 receptors are necessary for fear extinction and extinction-related tone responses. Biol Psychiatry 68(11):1055–1060
Dubrovina NI, Zinov’eva DV (2010) Effects of activation and blockade of dopamine receptors on the extinction of a passive avoidance reaction in mice with a depressive-like state. Neurosci Behav Physiol 40(1):55–59
Simón VM, Parra A, Miñarro J, Arenas MC, Vinader-Caerols C, Aguilar MA (2000) Predicting how equipotent doses of chlorpromazine, haloperidol, sulpiride, raclopride and clozapine reduce locomotor activity in mice. Eur Neuropsychopharmacol 10(3):159–164
Csernansky JG, Csernansky CA, Hollister LE (1985) 3[H]-Sulpiride labels mesolimbic non-dopaminergic sites that bind antidepressant drugs. Experientia 41(11):1419–1421
Lissek S, Glaubitz B, Wolf OT, Tegenthoff M (2015) The DA antagonist tiapride impairs context-related extinction learning in a novel context without affecting renewal. Front Behav Neurosci 9:238
Naylor JC, Kilts JD, Bradford DW, Strauss JL, Capehart BP, Szabo ST, Smith KD, Dunn CE et al (2015) A pilot randomized placebo-controlled trial of adjunctive aripiprazole for chronic PTSD in US military Veterans resistant to antidepressant treatment. Int Clin Psychopharmacol 30(3):167–174
Youssef NA, Marx CE, Bradford DW, Zinn S, Hertzberg MA, Kilts JD, Naylor JC, Butterfield MI et al (2012) An open-label pilot study of aripiprazole for male and female veterans with chronic post-traumatic stress disorder who respond suboptimally to antidepressants. Int Clin Psychopharmacol 27(4):191–196
Erman M, Seiden D, Zammit G, Sainati S, Zhang J (2006) An efficacy, safety, and dose-response study of ramelteon in patients with chronic primary insomnia. Sleep Med 7(1):17–24
Borja NL, Daniel KL (2006) Ramelteon for the treatment of insomnia. Clin Ther 28(10):1540–1555
Acknowledgments
This work was supported in part by grants-in-aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan (Kakenhi 25293124 and 26102704 to K.F.; Kakenhi 15H06036 to Y.Y.), the Uehara Memorial Foundation (K.F.), the Smoking Research Foundation (K.F.), and the Takeda Science Foundation (to Y.Y.). This research is partially supported by the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and Development, AMED (to K.F.).
Author information
Authors and Affiliations
Contributions
Y.Y. and K.F. designed the study and wrote the manuscript. Y.Y., I.T., and K.M. performed experiments. Y.O. provided Fabp3 −/− mice.
Corresponding author
Ethics declarations
All experimental procedures using animals were approved by the Committee on Animal Experiments at Tohoku University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yabuki, Y., Takahata, I., Matsuo, K. et al. Ramelteon Improves Post-traumatic Stress Disorder-Like Behaviors Exhibited by Fatty Acid-Binding Protein 3 Null Mice. Mol Neurobiol 55, 3577–3591 (2018). https://doi.org/10.1007/s12035-017-0587-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0587-2