Abstract
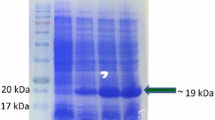
Calanthe mild mosaic virus (CalMMV) infecting orchids is an important potyvirus which is known to cause mild leaf mosaic and flower colour-breaking symptoms in Calanthe and other orchid plants. The present study reports the production of polyclonal antibodies against CalMMV using bacterially expressed recombinant coat protein as immunogen, which in turn would be useful in routine indexing and screening of orchid germplasm. The coat protein (CP) gene (~ 807 bp) of CalMMV isolated from infected orchid sample was cloned in expression vector, pET-28a ( +) that yielded ~ 31 kDa fusion protein with Histidine tag (His6BP). The expression of fusion CP was confirmed through SDS–PAGE and Western blotting. The His6BP-CalMMV-CP obtained in soluble state after purification was used to immunize New Zealand white rabbit for the production of polyclonal antibodies (PAb). The PAb produced against the purified fusion protein successfully detected CAlMMV in the orchid samples at a dilution of 1:2000 in direct antigen-coated enzyme-linked immunosorbent assay (DAC-ELISA). This study presents the first report of Histidine tag (His6BP) fusion CalMMV-CP-based antibody production and its successful application in the identification of the virus in orchid plants. Outcome of this study will be helpful in routine certification programmes, screening of orchid germplasm and production of CalMMV-free planting materials of orchids.






Similar content being viewed by others
Data Availability
The data generated or analyzed along with supplementary information which is included in the manuscript can be obtained from the corresponding author on reasonable request.
References
Christenhusz, M. J., & Byng, J. W. (2016). The number of known plants species in the world and its annual increase. Phytotaxa, 261, 201–217.
Singh, S. K., Agrawala, D. K., Lalal, J. S., Sudhanshu, S. D., Mao, A. A., & Singh, P. (2019). Orchids of India: A Pictorial Guide (p. 548). Botanical Survey of India.
Hew, C. S., Soh, W. P., & Ng, C. K. Y. (1998). Variation in photosynthetic characteristics along the leaf blade of Oncidium goldiana, a C3 tropical epiphytic orchid hybrid. International Journal of plant sciences, 159, 116–120.
Lee, C. H., Zheng, Y. X., & Jan, F. J. (2017). The orchid infecting viruses found in the 21st century. Orchid Biotechnology. https://doi.org/10.1142/9789813109223_0009
Pant, R. P., Rashmi, E. R., Manjunath, N., & Baranwal, V. K. (2020). Status of orchid viruses in India and management strategies for them. Applied Plant Virology. https://doi.org/10.1016/b978-0-12-818654-1.00051-7
Zettler, F. W., Ko, N. J., Wisler, G. C., Elliott, M. S., & Wong, S. M. (1990). Viruses of orchids and their control. Plant Disease, 74, 621–626. https://doi.org/10.1094/pd-74-0621
Pant, R. P., Basavaraj, Y. B., Srivastava, N., Bhattarai, A., Kumar, A., Baranwal, V. K., Sailo, N., Rampal, & Barman, D. (2019). First report of groundnut bud necrosis virus infecting Phalaenopsis in India. New Disease Reports, 39, 17.
Pant, R. P., Kapoor, R., Kumar, S., Srivastava, N., Kumar, M., & Baranwal, V. K. (2017). First report of mild mosaic in ground orchid, Phaius tankervilleae in India associated with infection of Calanthe mild mosaic virus. Plant Disease, 101, 1960.
Hull, R. (2002). Matthews’ Plant Virology (4th ed.). Elsevier.
Gibbs, A. J., Varma, A., & Woods, R. D. (1966). Viruses occurring in white clover (Trifolium repens) from permanent pastures in Britain. Annals of Applied Biology, 58, 231–240.
Milne, R. G., & Lesemann, D. E. (1984). Immunosorbent electron microscopy in plant virus studies. Methods in Virology, 8, 85–101.
Gibbs, A., & Mackenze, A. (1997). A primer pair for amplifying part of the genome of all potyvirids by RT-PCR. Journal of Virological Methods, 63, 9–16.
Sambrook, J., & Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual (p. 112). Cold Spring Harbor Laboratory.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of the bacterlophage T4. Nature, 227, 680–685.
Clark, M. F., & Adams, A. N. (1977). Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. Journal of General Virology, 34, 475–483. https://doi.org/10.1099/0022-1317-34-3-475
Clark, M. F., & Bar-Joseph, M. (1984). Enzyme immunosorbent assays in plant virology. Methods in Virology, 7, 51–85.
Ali, R. N., Dann, A. L., Cross, P. A., & Wilson, C. R. (2014). Multiplex RT-PCR detection of three common viruses infecting orchids. Archives of Virology, 159, 3095–3099. https://doi.org/10.1007/s00705-014-2161-9
Bhat, A. I., Bhadramurthy, V., Siju, S., & Hareesh, P. S. (2006). Detection and identification of Cymbidium mosaic virus infecting vanilla (Vanilla planifolia Andrews) in India based on coat protein gene sequence relationships. Journal of Plant Biochemistry and Biotechnology, 15, 33–37.
Pant, R. P., Das, M., Pun, K. B., Ramachandran, P., & Medhi, R. P. (2010). Occurrence of cymbidium mosaic and odontoglossum ringspot viruses in orchid germplasm of Sikkim and Darjeeling hills, their identification and diagnosis. Indian Phytopathology, 63, 326–332.
Sherpa, A. R., Bag, T. K., Hallan, V., Pathak, P., & Zaidi, A. A. (2006). The detection of odontoglossum ringspot virus in orchids from Sikkim, India. Australasian Plant Pathology, 35, 69–71.
Gara, I. W., Kondo, H., Maeda, T., Inouye, N., & Tamada, T. (1998). Calanthe mild mosaic virus, a new potyvirus causing a mild mosaic disease of Calanthe orchid in Japan. Journal of Phytopathology, 146, 357–363.
Singh, M. K., Sherpa, A. R., Hallan, V., & Zaidi, A. A. (2007). A potyvirus in Cymbidium spp. in Northern India. Australasian Plant Disease Notes., 2, 11–13.
Digangi, B. A., Gray, L. K., Levy, J. K., Dubovi, E. J., & Tucker, S. J. (2011). Detection of protective antibody titers against feline panleukopenia virus, feline herpesvirus-1, and feline calicivirus in shelter cats using a point-of-care ELISA. Journal of Feline Medicine and Surgery, 13, 912–918. https://doi.org/10.1016/j.jfms.2011.07.0094
Kim, S. J., Park, Y. H., & Park, K. T. (2020). Development of a novel reverse transcription PCR and its application to field sample testing for feline calicivirus prevalence in healthy stray cats in Korean. Journal of veterinary Science, 21(5), e71. https://doi.org/10.4142/jvs.2020.21.e7
Schaad, N. W., Frederick, R. D., Shaw, J., Schneider, W. L., Hickson, R., Petrillo, M. D., & Luster, D. G. (2003). Advances in molecular-based diagnostics in meeting crop biosecurity and phytosanitary issues. Annual Review of Phytopathology, 41, 305–324. https://doi.org/10.1146/annurev.phyto.41.052002.0954355
Streck, A. F., Rüster, D., Truyen, U., & Homeier, T. (2013). An updated TaqMan real-time PCR for canine and feline parvoviruses. Journal of Virological Methods, 193, 6–8. https://doi.org/10.1016/j.jviromet.2013.04.0255
Boscia, D., Greif, C., Gugerli, P., Martelli, G. P., Walter, B., & Gonsalves, D. (1995). Nomenclature of grapevine leafroll-associated putative closteroviruses. Vitis, 34, 171–175.
Ling, K.-S., Zhu, H. Y., & Gonsalves, D. (2004). Complete nucleotide sequence and genome organization of Grapevine leafroll-associated virus 3, type member of the genus Ampelovirus. Journal of General Virology, 85, 2099–2102.
Kamo, K., Jordan, R., Guaragna, M. A., Hsu, H. T., & Uneg, P. (2010). Resistance to cucumber mosaic virus in gladiolus plants transformed with either a defective replicase or coat protein subgroup II gene from cucumber mosaic virus. Plant Cell Reports, 29, 695–704. https://doi.org/10.1007/s00299-010-0855-3
Kumari, S. G., Makkouk, K. M., Katul, L., & Vetten, H. J. (2001). Polyclonal antibodies to the bacterially expressed coat protein of Faba bean necrotic yellows virus. Journal of Phytopathology, 149, 543–550. https://doi.org/10.1046/j.1439-0434.2001.00674.x
Rani, P., Pant, R. P., & Jain, R. K. (2010). Serological detection of cymbidium mosaic and Odontoglossum ringspot viruses in orchids with polyclonal antibodies produced against their recombinant coat proteins. Journal of Phytopathology., 158, 542–545.
Bowden, G. A., Paredes, A. M., & Georgiou, G. (1991). Structure and morphology of protein inclusion bodies in Escherichia coli. Biotechnology, 8, 725–730.
Khan, S., Jan, A. T., Mandal, B., & Haq, Q. M. (2012). Immunodiagnostics of cucumber mosaic virus using antisera developed against recombinant coat protein. Archives of Phytopathology and Plant Protection, 45, 561–569. https://doi.org/10.1080/03235408.2011.588043
Raikhy, R. G., Hallan, V., Kulshrestha, S., & Zaidi, A. A. (2007). Polyclonal antibodies to the coat protein of carnation etched ring virus expressed in bacterial system: Production and use in immunodiagnosis. Journal of Phytopathology, 155, 616–622.
Abdelkader, H. S., Abdel-Salam, A. M., El Saghir, S. M., & Hossein, M. H. (2004). Molecular cloning and expression of recombinant coat protein gene of banana bunchy top virus in E. coli and its use in the production of diagnostic antibodies. Arab Journal of Biotechnology, 7, 173–188.
Jain, R. K., Pandey, A. N., Krishnareddy, M., & Mandal, B. (2005). Immunodiagnosis of groundnut and watermelon bud necrosis viruses using polyclonal antiserum to recombinant nucleocapsid protein of groundnut bud necrosis virus. Journal of Virological Methods, 130, 162–164.
Rai, R., Khurana, S. P., Kumar, S., Gupta, N., & Baranwal, V. K. (2018). Serological detection of grapevine leafroll-associated virus 4 in grapevine growing areas of India using polyclonal antiserum raised against the recombinant coat protein. Crop Protection, 109, 128–135. https://doi.org/10.1016/j.cropro.2018.03.008
Kumar, R., Pant, R. P., Kapoor, S., Khar, A., & Baranwal, V. K. (2021). Development of polyclonal antibodies using bacterially expressed recombinant coat protein for the detection of onion yellow dwarf virus (OYDV) and identification of virus free onion genotypes. 3 Biotech, 11(8), 388.
Kapoor, R., Mandal, B., Paul, P. K., Chigurupati, P., & Jain, R. K. (2014). Production of cocktail of polyclonal antibodies using bacterial expressed recombinant protein for multiple virus detection. Journal of Virological Methods, 196, 7–14. https://doi.org/10.1016/j.jviromet.2013.09.016
Lee, S. C., & Cang, Y. C. (2008). Performances and application of antisera produced by recombinant capsid proteins of cymbidium mosaic virus and odontoglossum ring spot virus. European Journal of Plant Pathology, 122, 297–306. https://doi.org/10.1007/s10658-008-9293-2
Funding
This work was sponsored and funded under the “National Certification System for Tissue Culture Raised Plants (NCS-TCP)” programme which is currently running at Referral Centre for Virus Indexing, ICAR-IARI, New Delhi, by the Department of Biotechnology, Ministry of Science & Technology, Government of India.
Author information
Authors and Affiliations
Contributions
Conceptualization, NS, RK-2 (RK), RPP and VKB; Methodology, NS, RK-1 (Rakesh Kumar) and AK; Validation, NS, RK-1 and AK; Formal Analysis, NS, RK-2, SKS, NG. Investigation, NS, RK-1 and AK; Resources, RPP and VKB; Writing—Original Draft Preparation, NS, GK, SKS and NG; Writing—Review and Editing, SKS, RPP and VKB; Supervision, RPP and VKB; Funding Acquisition, RPP and VKB.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that this is an innovative work that has not been published in other research journal and they have no conflict of interest between them or personal relationship that might alter the research work summarized in the manuscript. All the authors contributed outstandingly to the work.
Ethical Approval
Not Applicable.
Informed Consent
Not Applicable.
Additional information
Publisher's Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Srivastava, N., Kumar, R., Kapoor, R. et al. Development of Polyclonal Antibodies-Based Serological Method for the Detection of Calanthe Mild Mosaic Virus and Application in Virus Certification Programme. Mol Biotechnol (2024). https://doi.org/10.1007/s12033-024-01074-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-024-01074-0