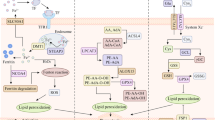
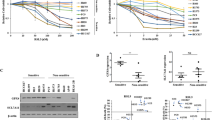
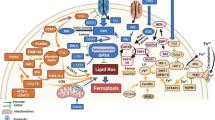
Abstract
Lung cancer has garnered significant global attention as a result of its escalating rates of mortality and morbidity, necessitating focused interventions to mitigate its impact. The primary aim of this work was to investigate the anticancer activity of juglone in A549 cells, specifically focusing on its role in mediating ferroptosis. We conducted an investigation involving a range of cytotoxic and morphological assays, such as cell viability assay, fluorescence microscopic analysis, flow cytometry, and ROS assay. The findings demonstrated that the cytotoxicity of juglone was around 18.5 μM. Furthermore, the chemical was found to promote apoptotic activity as observed through fluorescent microscopic inspection and morphological analysis. In addition, the levels of ROS, MDA, GSH, ferrous iron, and colony formation study demonstrated a significant increase, indicating a correlation with the occurrence of ferroptosis. Hence, juglone exhibits promise as a prospective therapeutic drug in the treatment of lung cancer. Therefore, we put forward that the utilization of ferroptosis as a therapeutic approach for lung cancer may yield significant efficacy and warrants further investigation in subsequent studies.











Similar content being viewed by others
Data Availability
Not applicable.
References
Khafaei, M., Miri, A., Kiani, E., Danesh, E., & Naderi, M. (2021). Early diagnostic biomarkers of lung cancer: A review study. Central Asian Journal of Medical and Pharmaceutical Sciences Innovation, 1(3), 114–130. https://doi.org/10.22034/CAJMPSI.2021.03.02
Thandra, K. C., Barsouk, A., Saginala, K., Aluru, J. S., & Barsouk, A. (2021). Epidemiology of lung cancer. Contemporary Oncology (Pozn). https://doi.org/10.5114/wo.2021.103829
Hassannia, B., Vandenabeele, P., & Vanden Berghe, T. (2019). Targeting ferroptosis to iron out cancer. Cancer Cell. https://doi.org/10.1016/j.ccell.2019.04.002
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., Patel, D. N., Bauer, A. J., Cantley, A. M., Yang, W. S., & Morrison, B. (2012). Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell, 149(5), 1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Yang, W. S., & Stockwell, B. R. (2008). Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chemistry and Biology, 15(3), 234–245. https://doi.org/10.1016/j.chembiol.2008.02.010
Brigelius-Flohé, R., & Maiorino, M. (2013). Glutathione peroxidases. Biochimica et Biophysica Acta General Subjects. https://doi.org/10.1016/j.bbagen.2012.11.020
Hassannia, B., Wiernicki, B., Ingold, I., Qu, F., Van Herck, S., Tyurina, Y. Y., & Berghe, T. V. (2018). Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. Journal of Clinical Investigation, 128(8), 3341–3355. https://doi.org/10.1172/JCI99032
Han, L., Lin, Q., Liu, G., Han, D., Niu, L., & Su, D. (2019). Catechin inhibits glycated phosphatidylethanolamine formation by trap** dicarbonyl compounds and forming quinone. Food and Function, 10(5), 2491–2503. https://doi.org/10.1039/c9fo00155g
Hyun, D. H. (2020). Insights into the new cancer therapy through redox homeostasis and metabolic shifts. Cancers, 12(7), 1–18. https://doi.org/10.3390/cancers12071822
Luo, Q., Hu, K., Chen, F., Gan, F.-J., Leng, Y.-X., Chen, X.-M., & Sun, S.-H. (2019). Juglone induces michigan cancer foundation-7 human breast cancer cells apoptosis through Bcl-2-associated X protein/B-cell lymphoma/leukemia-2 signal Way. Pharmacognosy Magazine, 15(65), 573. https://doi.org/10.4103/pm.pm_604_18
Ahmad, T., & Suzuki, Y. J. (2019). Juglone in oxidative stress and cell signaling. Antioxidants. https://doi.org/10.3390/antiox8040091
Wang, X., Hua, P., He, C., & Chen, M. (2022). Non-apoptotic cell death-based cancer therapy: Molecular mechanism, pharmacological modulators, and nanomedicine. Acta Pharmaceutica Sinica. B, 12(9), 3567–3593.
Kavithaa, K., Paulpandi, M., Ramya, S., Ramesh, M., Balachandar, V., Ramasamy, K., & Narayanasamy, A. (2021). Sitosterol-fabricated chitosan nanocomplex induces apoptotic cell death through mitochondrial dysfunction in lung cancer animal model: An enhanced synergetic drug delivery system for lung cancer therapy. New Journal of Chemistry, 45(20), 9251–9263. https://doi.org/10.1039/d1nj00913c
Ramya, S., Paulpandi, M., Kavithaa, K., Saranya, T., Winster, H., Balachandar, V., & Narayanasamy, A. (2021). Fabatin-loaded silica nanoparticle-induced apoptosisviamitochondrial dysfunction: Targeting the PI3K/AKT molecular pathway as a therapeutic implication against triple negative breast cancer. New Journal of Chemistry, 45(38), 17847–17861. https://doi.org/10.1039/d1nj02922c
Chen, P., Wu, Q., Feng, J., Yan, L., Sun, Y., Liu, S., **ang, Y., Zhang, M., Pan, T., Chen, X., & Duan, T. (2020). Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduction and Targeted Therapy, 5(1), 1–11. https://doi.org/10.1038/s41392-020-0149-3
Saranya, T., Kavithaa, K., Paulpandi, M., Ramya, S., Preethi, S., Balachandar, V., & Narayanasamy, A. (2020). Enhanced apoptogenesis and oncogene regulatory mechanism of troxerutin in triple negative breast cancer cells. Toxicology Research, 9(3), 230–238. https://doi.org/10.1093/TOXRES/TFAA029
Holohan, C., Van Schaeybroeck, S., Longley, D. B., & Johnston, P. G. (2013). Cancer drug resistance: An evolving paradigm. Nature Reviews Cancer. https://doi.org/10.1038/nrc3599
Su, Z., Yang, Z., **e, L., Dewitt, J. P., & Chen, Y. (2016). Cancer therapy in the necroptosis era. Cell Death and Differentiation. https://doi.org/10.1038/cdd.2016.8
Okada, H., & Mak, T. W. (2004). Pathways of apoptotic and non-apoptotic death in tumour cells. Nature Reviews Cancer. https://doi.org/10.1038/nrc1412
Zhang, Y. Y., Ni, Z. J., Elam, E., Zhang, F., Thakur, K., Wang, S., Zhang, J. G., & Wei, Z. J. (2021). Juglone, a novel activator of ferroptosis, induces cell death in endometrial carcinoma Ishikawa cells. Food and Function, 12(11), 4947–4959. https://doi.org/10.1039/d1fo00790d
Kiran Aithal, B., Sunil Kumar, M. R., Nageshwar Rao, B., Udupa, N., & Satish Rao, B. S. (2009). Juglone, a naphthoquinone from walnut, exerts cytotoxic and genotoxic effects against cultured melanoma tumor cells. Cell Biology International, 33(10), 1039–1049. https://doi.org/10.1016/j.cellbi.2009.06.018
Srinivas, P., Gopinath, G., Banerji, A., Dinakar, A., & Srinivas, G. (2004). Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Molecular Carcinogenesis, 40(4), 201–211. https://doi.org/10.1002/mc.20031
Sharma, V., Anderson, D., & Dhawan, A. (2012). Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis, 17(8), 852–870. https://doi.org/10.1007/s10495-012-0705-6
Dadashpour, M., Firouzi-Amandi, A., Pourhassan-Moghaddam, M., Maleki, M. J., Soozangar, N., Jeddi, F., Nouri, M., Zarghami, N., & Pilehvar-Soltanahmadi, Y. (2018). Biomimetic synthesis of silver nanoparticles using Matricaria chamomilla extract and their potential anticancer activity against human lung cancer cells. Materials Science and Engineering C, 92, 902–912. https://doi.org/10.1016/j.msec.2018.07.053
Sun, Y., Zheng, Y., Wang, C., & Liu, Y. (2018). Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells article. Cell Death and Disease, 9(7), 1–15. https://doi.org/10.1038/s41419-018-0794-4
Conrad, M., Kagan, V. E., Bayir, H., Pagnussat, G. C., Head, B., Traber, M. G., & Stockwell, B. R. (2018). Regulation of lipid peroxidation and ferroptosis in diverse species. Genes and Development. https://doi.org/10.1101/gad.314674.118
Imai, H., Matsuoka, M., Kumagai, T., Sakamoto, T., & Koumura, T. (2017). Lipid peroxidation-dependent cell death regulated by GPX4 and ferroptosis. Current Topics in Microbiology and Immunology, 403, 143–170. https://doi.org/10.1007/82_2016_508
Ma, S., Henson, E. S., Chen, Y., & Gibson, S. B. (2016). Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death & Disease, 7(7), e2307. https://doi.org/10.1038/cddis.2016.208
Mou, Y., Wang, J., Wu, J., He, D., Zhang, C., Duan, C., & Li, B. (2019). Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. Journal of Hematology and Oncology. https://doi.org/10.1186/s13045-019-0720-y
Liang, H., Yoo, S. E., Na, R., Walter, C. A., Richardson, A., & Ran, Q. (2009). Ran, Short form glutathione peroxidase 4 is the essential isoform required for survival and somatic mitochondrial functions. Journal of Biological Chemistry, 284, 30836.
Ni, J., Chen, K., Zhang, J., & Zhang, X. (2021). Inhibition of GPX4 or mTOR overcomes resistance to Lapatinib via promoting ferroptosis in NSCLC cells. Biochemical and Biophysical Research Communications, 567, 154–160. https://doi.org/10.1016/j.bbrc.2021.06.051
Khoubnasab Jafari, M., Ansarin, K., & Jouyban, A. (2015). Comments on “Use of malondialdehyde as a biomarker for assesing oxidative stress in different disease pathologies: A review.” Iranian Journal of Public Health., 44(5), 714–715.
Hou, W., **e, Y., Song, X., Sun, X., Lotze, M. T., Zeh, H. J., Kang, R., & Tang, D. (2016). Autophagy promotes ferroptosis by degradation of ferritin. Autophagy, 12, 1425–1428.
Song, X., Zhu, S., Chen, P., Hou, W., Wen, Q., Liu, J., **e, Y., Liu, J., Klionsky, D. J., Kroemer, G., Lotze, M. T., Zeh, H. J., Kang, R., & Tang, D. (2018). AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system xcactivity. Current Biology, 28, 2388–2399.
Du, Y., Zhao, H. C., Zhu, H. C., **, Y., & Wang, L. (2021). Ferroptosis is involved in the anti-tumor effect of lycorine in renal cell carcinoma cells. Oncology Letters. https://doi.org/10.3892/ol.2021.13042
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
JD and DY— involved in manuscript preparation and working the scientific part of the manuscript. DY framed the work and revised the manuscript for submission.
Corresponding author
Ethics declarations
Competing Interests
All the authors had declared no conflict of interest.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, J., Krishnamoorthy, K., Ramabhai, V. et al. Targeting Ferroptosis as a Therapeutic Implication in Lung Cancer Treatment by a Novel Naphthoquinone Inducer: Juglone. Mol Biotechnol 66, 1071–1081 (2024). https://doi.org/10.1007/s12033-023-01004-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-01004-6