Abstract
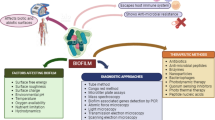
Biofilms are considered as a severe problem in the treatment of bacterial infections; their development causes some noticeable resistance to antibacterial agents. Biofilms are responsible for at least two-thirds of all infections, displaying promoted resistance to classical antibiotic treatments. Therefore, finding new alternative therapeutic approaches is essential for the treatment and inhibition of biofilm-related infections. Therefore, this review aims to describe the potential therapeutic strategies that can inhibit bacterial biofilm development; these include the usage of antiadhesion agents, AMPs, bacteriophages, QSIs, aptamers, NPs and PNAs, which can prevent or eradicate the formation of biofilms. These antibiofilm agents represent a promising therapeutic target in the treatment of biofilm infections and development of a strong capability to interfere with different phases of the biofilm development, including adherence, polysaccharide intercellular adhesion (PIA), quorum sensing molecules and cell-to-cell connection, bacterial aggregation, planktonic bacteria killing and host-immune response modulation. In addition, these components, in combination with antibiotics, can lead to the development of some kind of powerful combined therapy against bacterial biofilm-related infections.



Similar content being viewed by others
References
Høiby, N., Bjarnsholt, T., Moser, C., Bassi, G., Coenye, T., Donelli, G., Hall-Stoodley, L., Holá, V., Imbert, C., & Kirketerp-Møller, K. (2015). ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clinical Microbiology & Infection, 21, S1–S25.
Evans, J. J., & Bolz, D. D. (2019). Regulation of virulence and antibiotic resistance in Gram-positive microbes in response to cell wall-active antibiotics. Current Opinion in Infectious Diseases, 32, 217–222.
Khatoon, Z., McTiernan, C. D., Suuronen, E. J., Mah, T.-F., & Alarcon, E. I. (2018). Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon, 4, e01067.
Batoni, G., Maisetta, G., & Esin, S. (2016). Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1858, 1044–1060.
Lynch, A. S., & Robertson, G. T. (2008). Bacterial and fungal biofilm infections. Annual Review of Medicine, 59, 415–428.
Hall-Stoodley, L., Costerton, J. W., & Stoodley, P. (2004). Bacterial biofilms: From the natural environment to infectious diseases. Nature Reviews Microbiology, 2, 95–108.
Costerton, J. W., Cheng, K., Geesey, G. G., Ladd, T. I., Nickel, J. C., Dasgupta, M., & Marrie, T. J. (1987). Bacterial biofilms in nature and disease. Annual Reviews in Microbiology, 41, 435–464.
Lei, M. G., Gupta, R. K., & Lee, C. Y. (2017). Proteomics of Staphylococcus aureus biofilm matrix in a rat model of orthopedic implant-associated infection. PLoS ONE, 12, e0187981.
Watnick, P., & Kolter, R. (2000). Biofilm, city of microbes. Journal of Bacteriology, 182, 2675–2679.
Galdiero, E., Lombardi, L., Falanga, A., Libralato, G., Guida, M., & Carotenuto, R. (2019). Biofilms: Novel strategies based on antimicrobial peptides. Pharmaceutics, 11, 322.
Staudt, C., Horn, H., Hempel, D., & Neu, T. (2004). Volumetric measurements of bacterial cells and extracellular polymeric substance glycoconjugates in biofilms. Biotechnology and Bioengineering, 88, 585–592.
Dibdin, G. H., Assinder, S. J., Nichols, W. W., & Lambert, P. A. (1996). Mathematical model of β-lactam penetration into a biofilm of Pseudomonas aeruginosa while undergoing simultaneous inactivation by released β-lactamases. Journal of Antimicrobial Chemotherapy, 38, 757–769.
Bhagirath, A. Y., Li, Y., Somayajula, D., Dadashi, M., Badr, S., & Duan, K. (2016). Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulmonary Medicine, 16, 1–22.
Ebbensgaard, A., Mordhorst, H., Overgaard, M. T., Nielsen, C. G., Aarestrup, F. M., & Hansen, E. B. (2015). Comparative evaluation of the antimicrobial activity of different antimicrobial peptides against a range of pathogenic bacteria. PLoS ONE, 10, e0144611.
Kåhrström, C. T. (2013). Entering a post-antibiotic era? Nature Reviews Microbiology, 11, 146–146.
Di Somma, A., Moretta, A., Canè, C., Cirillo, A., & Duilio, A. (2020). Antimicrobial and antibiofilm peptides. Biomolecules, 10, 652.
Ferriol-González, C., & Domingo-Calap, P. (2020). Phages for biofilm removal. Antibiotics, 9, 268.
Haque, S., Ahmad, F., Dar, S. A., Jawed, A., Mandal, R. K., Wahid, M., Lohani, M., Khan, S., Singh, V., & Akhter, N. (2018). Developments in strategies for Quorum Sensing virulence factor inhibition to combat bacterial drug resistance. Microbial Pathogenesis, 121, 293–302.
Shatila, F., Yaşa, İ, & Yalçın, H. (2020). Inhibition of Salmonella enteritidis biofilms by Salmonella invasion protein-targeting aptamer. Biotechnology Letters. https://doi.org/10.1007/s10529-020-02920-2
Fulaz, S., Vitale, S., Quinn, L., & Casey, E. (2019). Nanoparticle–biofilm interactions: The role of the EPS matrix. Trends in Microbiology, 27, 915–926.
Narenji, H., Teymournejad, O., Rezaee, M. A., Taghizadeh, S., Mehramuz, B., Aghazadeh, M., Asgharzadeh, M., Madhi, M., Gholizadeh, P., Ganbarov, K., Yousefi, M., Pakravan, A., Dal, T., Ahmadi, R., & Samadi Kafil, H. (2020). Antisense peptide nucleic acids againstftsZ andefaA genes inhibit growth and biofilm formation of Enterococcus faecalis. Microbial Pathogenesis, 139, 103907.
Arias, C. A., & Murray, B. E. (2009). Antibiotic-resistant bugs in the 21st century—A clinical super-challenge. New England Journal of Medicine, 360, 439–443.
Wood, L. F., Leech, A. J., & Ohman, D. E. (2006). Cell wall-inhibitory antibiotics activate the alginate biosynthesis operon in Pseudomonas aeruginosa: roles of σ22 (AlgT) and the AlgW and Prc proteases. Molecular Microbiology, 62, 412–426.
Wiens, J. R., Vasil, A. I., Schurr, M. J., & Vasil, M. L. (2014). Iron-regulated expression of alginate production, mucoid phenotype, and biofilm formation by Pseudomonas aeruginosa. MBio. https://doi.org/10.1128/mBio.01010-13
Bhattacharyya, P., Agarwal, B., Goswami, M., Maiti, D., Baruah, S., & Tribedi, P. (2018). Zinc oxide nanoparticle inhibits the biofilm formation of Streptococcus pneumoniae. Antonie van Leeuwenhoek, 111, 89–99.
Ali, S. G., Ansari, M. A., Alzohairy, M. A., Alomary, M. N., AlYahya, S., Jalal, M., Khan, H. M., Asiri, S. M. M., Ahmad, W., & Mahdi, A. A. (2020). Biogenic gold nanoparticles as potent antibacterial and antibiofilm nano-antibiotics against Pseudomonas aeruginosa. Antibiotics, 9, 100.
Kim, H.-S., Lee, S.-H., Byun, Y., & Park, H.-D. (2015). 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Science and Reports, 5, 8656.
Bahari, S., Zeighami, H., Mirshahabi, H., Roudashti, S., & Haghi, F. (2017). Inhibition of Pseudomonas aeruginosa quorum sensing by subinhibitory concentrations of curcumin with gentamicin and azithromycin. Journal of Global Antimicrobial Resistance, 10, 21–28.
De la Fuente-Núñez, C., Reffuveille, F., Fernández, L., & Hancock, R. E. (2013). Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Current Opinion in Microbiology, 16, 580–589.
Donlan, R. M. (2002). Biofilms: Microbial life on surfaces. Emerging Infectious Diseases, 8, 881.
Beloin, C., Valle, J., Latour-Lambert, P., Faure, P., Kzreminski, M., Balestrino, D., Haagensen, J. A., Molin, S., Prensier, G., & Arbeille, B. (2004). Global impact of mature biofilm lifestyle on Escherichia coli K-12 gene expression. Molecular Microbiology, 51, 659–674.
Klausen, M., Aaes-Jørgensen, A., Molin, S., & Tolker-Nielsen, T. (2003). Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Molecular Microbiology, 50, 61–68.
Klausen, M., Heydorn, A., Ragas, P., Lambertsen, L., Aaes-Jørgensen, A., Molin, S., & Tolker-Nielsen, T. (2003). Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Molecular Microbiology, 48, 1511–1524.
Toutain, C. M., Caizza, N. C., Zegans, M. E., & O’Toole, G. A. (2007). Roles for flagellar stators in biofilm formation by Pseudomonas aeruginosa. Research in Microbiology, 158, 471–477.
Watnick, P. I., & Kolter, R. (1999). Steps in the development of a Vibrio cholerae El Tor biofilm. Molecular Microbiology, 34, 586–595.
Schmidt, J., Müsken, M., Becker, T., Magnowska, Z., Bertinetti, D., Möller, S., Zimmermann, B., Herberg, F. W., Jänsch, L., & Häussler, S. (2011). The Pseudomonas aeruginosa chemotaxis methyltransferase CheR1 impacts on bacterial surface sampling. PLoS ONE, 6, e18184.
Hadjifrangiskou, M., Gu, A. P., Pinkner, J. S., Kostakioti, M., Zhang, E. W., Greene, S. E., & Hultgren, S. J. (2012). Transposon mutagenesis identifies uropathogenic Escherichia coli biofilm factors. Journal of Bacteriology, 194, 6195–6205.
Beloin, C., Roux, A., & Ghigo, J.-M. (2008). Escherichia coli biofilms. In Bacterial biofilms. (pp. 249–289). Springer.
Anderson, G. G., Palermo, J. J., Schilling, J. D., Roth, R., Heuser, J., & Hultgren, S. J. (2003). Intracellular bacterial biofilm-like pods in urinary tract infections. Science, 301, 105–107.
Waksman, G., & Hultgren, S. J. (2009). Structural biology of the chaperone–usher pathway of pilus biogenesis. Nature Reviews Microbiology, 7, 765–774.
Spurbeck, R. R., Stapleton, A. E., Johnson, J. R., Walk, S. T., Hooton, T. M., & Mobley, H. L. (2011). Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: contribution of ygi and yad fimbriae. Infection and Immunity, 79, 4753–4763.
Thumbikat, P., Berry, R. E., Zhou, G., Billips, B. K., Yaggie, R. E., Zaichuk, T., Sun, T.-T., Schaeffer, A. J., & Klumpp, D. J. (2009). Bacteria-induced uroplakin signaling mediates bladder response to infection. PLoS Pathogens, 5, e1000415.
Cegelski, L., Pinkner, J. S., Hammer, N. D., Cusumano, C. K., Hung, C. S., Chorell, E., Åberg, V., Walker, J. N., Seed, P. C., & Almqvist, F. (2009). Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nature Chemical Biology, 5, 913–919.
Klebensberger, J., Birkenmaier, A., Geffers, R., Kjelleberg, S., & Philipp, B. (2009). SiaA and SiaD are essential for inducing autoaggregation as a specific response to detergent stress in Pseudomonas aeruginosa. Environmental Microbiology, 11, 3073–3086.
Najafi, K., Ganbarov, K., Gholizadeh, P., Tanomand, A., Rezaee, M. A., Mahmood, S. S., Asgharzadeh, M., & Kafil, H. S. (2020). Oral cavity infection by Enterococcus faecalis: Virulence factors and pathogenesis. Reviews in Medical Microbiology, 31, 51–60.
Sillanpää, J., Nallapareddy, S. R., Prakash, V. P., Qin, X., Hook, M., Weinstock, G. M., & Murray, B. E. (2008). Identification and phenotypic characterization of a second collagen adhesin, Scm, and genome-based identification and analysis of 13 other predicted MSCRAMMs, including four distinct pilus loci, in Enterococcus faecium. Microbiology (Reading, England), 154, 3199.
Han, Z., Pinkner, J. S., Ford, B., Obermann, R., Nolan, W., Wildman, S. A., Hobbs, D., Ellenberger, T., Cusumano, C. K., & Hultgren, S. J. (2010). Structure-based drug design and optimization of mannoside bacterial FimH antagonists. Journal of Medicinal Chemistry, 53, 4779–4792.
Bouckaert, J., Berglund, J., Schembri, M., De Genst, E., Cools, L., Wuhrer, M., Hung, C. S., Pinkner, J., Slättegård, R., & Zavialov, A. (2005). Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Molecular Microbiology, 55, 441–455.
Cusumano, C. K., Pinkner, J. S., Han, Z., Greene, S. E., Ford, B. A., Crowley, J. R., Henderson, J. P., Janetka, J. W., & Hultgren, S. J. (2011). Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Science Translational Medicine, 3, 109ra115.
Chorell, E., Pinkner, J. S., Phan, G., Edvinsson, S., Buelens, F., Remaut, H., Waksman, G., Hultgren, S. J., & Almqvist, F. (2010). Design and synthesis of C-2 substituted thiazolo and dihydrothiazolo ring-fused 2-pyridones: Pilicides with increased antivirulence activity. Journal of Medicinal Chemistry, 53, 5690–5695.
Chorell, E., Bengtsson, C., Banchelin, T.S.-L., Das, P., Uvell, H., Sinha, A. K., Pinkner, J. S., Hultgren, S. J., & Almqvist, F. (2011). Synthesis and application of a bromomethyl substituted scaffold to be used for efficient optimization of anti-virulence activity. European Journal of Medicinal Chemistry, 46, 1103–1116.
Yazici, A., Ortucu, S., Taskin, M., & Marinelli, L. (2018). Natural-based antibiofilm and antimicrobial peptides from micro-organisms. Current Topics in Medicinal Chemistry, 18, 2102–2107.
Ageitos, J., Sánchez-Pérez, A., Calo-Mata, P., & Villa, T. (2017). Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochemical Pharmacology, 133, 117–138.
Zhang, L., & Falla, T. J. (2006). Antimicrobial peptides: Therapeutic potential. Expert Opinion on Pharmacotherapy, 7, 653–663.
Yeaman, M. R., & Yount, N. Y. (2003). Mechanisms of antimicrobial peptide action and resistance. Pharmacological Reviews, 55, 27–55.
Jenssen, H., Hamill, P., & Hancock, R. E. (2006). Peptide antimicrobial agents. Clinical Microbiology Reviews, 19, 491–511.
Brogden, K. A. (2005). Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nature Reviews Microbiology, 3, 238–250.
Cassone, M., Frith, N., Vogiatzi, P., Wade, J. D., & Otvos, L. (2009). Induced resistance to the designer proline-rich antimicrobial peptide A3-APO does not involve changes in the intracellular target DnaK. International Journal of Peptide Research and Therapeutics, 15, 121–128.
Shah, P., Hsiao, F. S. H., Ho, Y. H., & Chen, C. S. (2016). The proteome targets of intracellular targeting antimicrobial peptides. Proteomics, 16, 1225–1237.
Graf, M., & Wilson, D. N. (2019). Intracellular antimicrobial peptides targeting the protein synthesis machinery. In Antimicrobial peptides. (pp. 73–89). Springer.
Okuda, K.-I., Zendo, T., Sugimoto, S., Iwase, T., Tajima, A., Yamada, S., Sonomoto, K., & Mizunoe, Y. (2013). Effects of bacteriocins on methicillin-resistant Staphylococcus aureus biofilm. Antimicrobial Agents and Chemotherapy, 57, 5572–5579.
Overhage, J., Campisano, A., Bains, M., Torfs, E. C., Rehm, B. H., & Hancock, R. E. (2008). Human host defense peptide LL-37 prevents bacterial biofilm formation. Infection and Immunity, 76, 4176–4182.
Brancatisano, F. L., Maisetta, G., Di Luca, M., Esin, S., Bottai, D., Bizzarri, R., Campa, M., & Batoni, G. (2014). Inhibitory effect of the human liver-derived antimicrobial peptide hepcidin 20 on biofilms of polysaccharide intercellular adhesin (PIA)-positive and PIA-negative strains of Staphylococcus epidermidis. Biofouling, 30, 435–446.
Blower, R. J., Barksdale, S. M., & van Hoek, M. L. (2015). Snake cathelicidin NA-CATH and smaller helical antimicrobial peptides are effective against Burkholderia thailandensis. PLoS Neglected Tropical Diseases, 9, e0003862.
Anunthawan, T., De La Fuente-Núñez, C., Hancock, R. E., & Klaynongsruang, S. (2015). Cationic amphipathic peptides KT2 and RT2 are taken up into bacterial cells and kill planktonic and biofilm bacteria. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1848, 1352–1358.
De Brucker, K., Delattin, N., Robijns, S., Steenackers, H., Verstraeten, N., Landuyt, B., Luyten, W., Schoofs, L., Dovgan, B., & Fröhlich, M. (2014). Derivatives of the mouse cathelicidin-related antimicrobial peptide (CRAMP) inhibit fungal and bacterial biofilm formation. Antimicrobial Agents and Chemotherapy, 58, 5395–5404.
Mataraci, E., & Dosler, S. (2012). In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrobial Agents and Chemotherapy, 56, 6366–6371.
Cao, Y., Yin, H., Wang, W., Pei, P., Wang, Y., Wang, X., Jiang, J., Luo, S.-Z., & Chen, L. (2020). Killing Streptococcus mutans in mature biofilm with a combination of antimicrobial and antibiofilm peptides. Amino Acids, 52, 1–14.
Gopal, R., Lee, J. H., Kim, Y. G., Kim, M.-S., Seo, C. H., & Park, Y. (2013). Anti-microbial, anti-biofilm activities and cell selectivity of the NRC-16 peptide derived from witch flounder, Glyptocephalus cynoglossus. Marine Drugs, 11, 1836–1852.
Chen, L., Jia, L., Zhang, Q., Zhou, X., Liu, Z., Li, B., Zhu, Z., Wang, F., Yu, C., & Zhang, Q. (2017). A novel antimicrobial peptide against dental-caries-associated bacteria. Anaerobe, 47, 165–172.
Cooper, V. S., Carlson, W. A., & LiPuma, J. J. (2009). Susceptibility of Caenorhabditis elegans to Burkholderia infection depends on prior diet and secreted bacterial attractants. PLoS ONE, 4, e7961.
Peng, X., Zhang, Y., Bai, G., Zhou, X., & Wu, H. (2016). Cyclic di-AMP mediates biofilm formation. Molecular Microbiology, 99, 945–959.
Pletzer, D., Coleman, S. R., & Hancock, R. E. (2016). Anti-biofilm peptides as a new weapon in antimicrobial warfare. Current Opinion in Microbiology, 33, 35–40.
Ribeiro, S. M., De La Fuente-Núñez, C., Baquir, B., Faria-Junior, C., Franco, O. L., & Hancock, R. E. (2015). Antibiofilm peptides increase the susceptibility of carbapenemase-producing Klebsiella pneumoniae clinical isolates to β-lactam antibiotics. Antimicrobial Agents and Chemotherapy, 59, 3906–3912.
Tong, Z., Zhang, Y., Ling, J., Ma, J., Huang, L., & Zhang, L. (2014). An in vitro study on the effects of nisin on the antibacterial activities of 18 antibiotics against Enterococcus faecalis. PLoS ONE, 9, e89209.
Tong, Z., Zhou, L., Jiang, W., Kuang, R., Li, J., Tao, R., & Ni, L. (2011). An in vitro synergetic evaluation of the use of nisin and sodium fluoride or chlorhexidine against Streptococcus mutans. Peptides, 32, 2021–2026.
Domingo-Calap, P., & Delgado-Martínez, J. (2018). Bacteriophages: Protagonists of a post-antibiotic era. Antibiotics, 7, 66.
Principi, N., Silvestri, E., & Esposito, S. (2019). Advantages and limitations of bacteriophages for the treatment of bacterial infections. Frontiers in Pharmacology, 10, 513.
Bedi, M. S., Verma, V., & Chhibber, S. (2009). Amoxicillin and specific bacteriophage can be used together for eradication of biofilm of Klebsiella pneumoniae B5055. World Journal of Microbiology & Biotechnology, 25, 1145.
Lu, T. K., & Collins, J. J. (2007). Dispersing biofilms with engineered enzymatic bacteriophage. Proceedings of the National Academy of Sciences, 104, 11197–11202.
Łusiak-Szelachowska, M., Weber-Dąbrowska, B., & Górski, A. (2020). Bacteriophages and lysins in biofilm control. Virologica Sinica. https://doi.org/10.1007/s12250-019-00192-3
Wood, S., Kirkham, J., Marsh, P., Shore, R., Nattress, B., & Robinson, C. (2000). Architecture of intact natural human plaque biofilms studied by confocal laser scanning microscopy. Journal of Dental Research, 79, 21–27.
Pires, D. P., Oliveira, H., Melo, L. D., Sillankorva, S., & Azeredo, J. (2016). Bacteriophage-encoded depolymerases: Their diversity and biotechnological applications. Applied Microbiology and Biotechnology, 100, 2141–2151.
Cornelissen, A., Ceyssens, P. J., T’syen, J., Van Praet, H., Noben, J. P., Shaburova, O. V., Krylov, V. N., Volckaert, G., & Lavigne, R. (2011). The T7-related Pseudomonas putida phage φ15 displays virion-associated biofilm degradation properties. PLoS ONE, 6, e18597.
Yoon, S., Choi, Y., Lee, S. Y., Son, J., Jun, S., & Kang, S. (2013) Bacteriophage or lytic protein derived from the bacteriophage which effective for the treatment of Staphylococcus aureus biofilm, Google Patents.
Borysowski, J., Łobocka, M., Międzybrodzki, R., Weber-Dąbrowska, B., & Górski, A. (2011). Potential of bacteriophages and their lysins in the treatment of MRSA. BioDrugs, 25, 347–355.
Fischetti, V. A. (2018). Development of phage lysins as novel therapeutics: A historical perspective. Viruses, 10, 310.
Sharma, U., Vipra, A., & Channabasappa, S. (2018). Phage-derived lysins as potential agents for eradicating biofilms and persisters. Drug Discovery Today, 23, 848–856.
Gray, J. A., Chandry, P. S., Kaur, M., Kocharunchitt, C., Bowman, J. P., & Fox, E. M. (2018). Novel biocontrol methods for Listeria monocytogenes biofilms in food production facilities. Frontiers in Microbiology, 9, 605.
Briers, Y., Walmagh, M., Grymonprez, B., Biebl, M., Pirnay, J.-P., Defraine, V., Michiels, J., Cenens, W., Aertsen, A., & Miller, S. (2014). Art-175 is a highly efficient antibacterial against multidrug-resistant strains and persisters of Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy, 58, 3774–3784.
Donlan, R. M. (2009). Preventing biofilms of clinically relevant organisms using bacteriophage. Trends in Microbiology, 17, 66–72.
Coulter, L. B., McLean, R. J., Rohde, R. E., & Aron, G. M. (2014). Effect of bacteriophage infection in combination with tobramycin on the emergence of resistance in Escherichia coli and Pseudomonas aeruginosa biofilms. Viruses, 6, 3778–3786.
Issa, R., Chanishvili, N., Caplin, J., Kakabadze, E., Bakuradze, N., Makalatia, K., & Cooper, I. (2019). Antibiofilm potential of purified environmental bacteriophage preparations against early stage Pseudomonas aeruginosa biofilms. Journal of Applied Microbiology, 126, 1657–1667.
Maciejewska, B., Olszak, T., & Drulis-Kawa, Z. (2018). Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: An ambitious and also a realistic application? Applied Microbiology and Biotechnology, 102, 2563–2581.
Abedon, S. T. (2019). Phage-antibiotic combination treatments: Antagonistic impacts of antibiotics on the pharmacodynamics of phage therapy? Antibiotics, 8, 182.
Tagliaferri, T. L., Jansen, M., & Horz, H.-P. (2019). Fighting pathogenic bacteria on two fronts: Phages and antibiotics as combined strategy. Frontiers in Cellular and Infection Microbiology, 9, 22.
Fuqua, C., Parsek, M. R., & Greenberg, E. P. (2001). Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Annual Review of Genetics, 35, 439–468.
Dijkshoorn, L., Nemec, A., & Seifert, H. (2007). An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nature Reviews Microbiology, 5, 939–951.
Hemmati, F., Salehi, R., Ghotaslou, R., Kafil, H. S., Hasani, A., Gholizadeh, P., Nouri, R., & Rezaee, M. A. (2020). Quorum Quenching: A potential target for antipseudomonal therapy. Infection and Drug Resistance, 13, 2989–3005.
Kalia, V. C. (2013). Quorum sensing inhibitors: An overview. Biotechnology Advances, 31, 224–245.
Dembitsky, V. M., Al Quntar, A. A. A., & Srebnik, M. (2011). Natural and synthetic small boron-containing molecules as potential inhibitors of bacterial and fungal quorum sensing. Chemical Reviews, 111, 209–237.
Kaufmann, G. F., Sartorio, R., Lee, S.-H., Mee, J. M., Altobell, L. J., Kujawa, D. P., Jeffries, E., Clapham, B., Meijler, M. M., & Janda, K. D. (2006). Antibody interference with N-acyl homoserine lactone-mediated bacterial quorum sensing. Journal of the American Chemical Society, 128, 2802–2803.
Bassler, B. L. (2002). Small talk: Cell-to-cell communication in bacteria. Cell, 109, 421–424.
Taylor, P. K., Yeung, A. T., & Hancock, R. E. (2014). Antibiotic resistance in Pseudomonas aeruginosa biofilms: Towards the development of novel anti-biofilm therapies. Journal of Biotechnology, 191, 121–130.
de Almeida, F. A., Vargas, E. L. G., Carneiro, D. G., Pinto, U. M., & Vanetti, M. C. D. (2018). Virtual screening of plant compounds and nonsteroidal anti-inflammatory drugs for inhibition of quorum sensing and biofilm formation in Salmonella. Microbial Pathogenesis, 121, 369–388.
Yang, E., Lu, Y., Xu, Y., Liang, Q., Wang, C., Wang, H., & Shen, H. (2014). Recombinant BCG coexpressing Ag85B, ESAT-6 and Rv3620c elicits specific Th1 immune responses in C57BL/6 mice. Microbial Pathogenesis, 69, 53–59.
Soheili, V., Bazzaz, B. S. F., Abdollahpour, N., & Hadizadeh, F. (2015). Investigation of Pseudomonas aeruginosa quorum-sensing signaling system for identifying multiple inhibitors using molecular docking and structural analysis methodology. Microbial Pathogenesis, 89, 73–78.
Skindersoe, M. E., Alhede, M., Phipps, R., Yang, L., Jensen, P. O., Rasmussen, T. B., Bjarnsholt, T., Tolker-Nielsen, T., Høiby, N., & Givskov, M. (2008). Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy, 52, 3648–3663.
Sofer, D., Gilboa-Garber, N., Belz, A., & Garber, N. C. (1999). ‘Subinhibitory’erythromycin represses production of Pseudomonas aeruginosa lectins, autoinducer and virulence factors. Chemotherapy, 45, 335–341.
Dong, Y.-H., & Zhang, L.-H. (2005). Quorum sensing and quorum-quenching enzymes. The Journal of Microbiology, 43, 101–109.
Chanda, S., & Rakholiya, K. (2011). Combination therapy: Synergism between natural plant extracts and antibiotics against infectious diseases. Microbiology Book Series, 1, 520–529.
Zhou, J.-W., Chen, T.-T., Tan, X.-J., Sheng, J.-Y., & Jia, A.-Q. (2018). Can the quorum sensing inhibitor resveratrol function as an aminoglycoside antibiotic accelerant against Pseudomonas aeruginosa? International Journal of Antimicrobial Agents, 52, 35–41.
Roudashti, S., Zeighami, H., Mirshahabi, H., Bahari, S., Soltani, A., & Haghi, F. (2017). Synergistic activity of sub-inhibitory concentrations of curcumin with ceftazidime and ciprofloxacin against Pseudomonas aeruginosa quorum sensing related genes and virulence traits. World Journal of Microbiology & Biotechnology, 33, 50.
Hemmati, F., Salehi, R., Ghotaslou, R., Kafil, H. S., Hasani, A., Gholizadeh, P., & Rezaee, M. A. (2020). The assessment of antibiofilm activity of chitosan-zinc oxide-gentamicin nanocomposite on Pseudomonas aeruginosa and Staphylococcus aureus. International Journal of Biological Macromolecules, 163, 2248–2258.
de Nys, R., Wright, A. D., König, G. M., & Sticher, O. (1993). New halogenated furanones from the marine alga Delisea pulchra (cf. fimbriata). Tetrahedron, 49, 11213–11220.
De Nys, R., Givskov, M., Kumar, N., Kjelleberg, S., & Steinberg, P. (2006). Furanones. In Antifouling compounds. (pp. 55–86). Springer.
Rasmussen, T. B., Skindersoe, M. E., Bjarnsholt, T., Phipps, R. K., Christensen, K. B., Jensen, P. O., Andersen, J. B., Koch, B., Larsen, T. O., & Hentzer, M. (2005). Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiology, 151, 1325–1340.
Musthafa, K. S., Ravi, A. V., Annapoorani, A., Packiavathy, I. S. V., & Pandian, S. K. (2010). Evaluation of anti-quorum-sensing activity of edible plants and fruits through inhibition of the N-acyl-homoserine lactone system in Chromobacterium violaceum and Pseudomonas aeruginosa. Chemotherapy, 56, 333–339.
Girennavar, B., Cepeda, M. L., Soni, K. A., Vikram, A., Jesudhasan, P., Jayaprakasha, G., Pillai, S. D., & Patil, B. S. (2008). Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. International Journal of Food Microbiology, 125, 204–208.
Adonizio, A., Kong, K.-F., & Mathee, K. (2008). Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrobial Agents and Chemotherapy, 52, 198–203.
Vandeputte, O. M., Kiendrebeogo, M., Rajaonson, S., Diallo, B., Mol, A., El Jaziri, M., & Baucher, M. (2010). Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Applied and Environment Microbiology, 76, 243–253.
Zhou, L., Zheng, H., Tang, Y., Yu, W., & Gong, Q. (2013). Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnology Letters, 35, 631–637.
Packiavathy, I. A. S. V., Priya, S., Pandian, S. K., & Ravi, A. V. (2014). Inhibition of biofilm development of uropathogens by curcumin—An anti-quorum sensing agent from Curcuma longa. Food Chemistry, 148, 453–460.
Burt, S. A., Ojo-Fakunle, V. T., Woertman, J., & Veldhuizen, E. J. (2014). The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE, 9, e93414.
Pe**, B., Ciric, A., Glamoclija, J., Nikolic, M., & Sokovic, M. (2015). In vitro anti-quorum sensing activity of phytol. Natural Product Research, 29, 374–377.
Husain, F. M., Ahmad, I., Khan, M. S., Ahmad, E., Tahseen, Q., Khan, M. S., & Alshabib, N. A. (2015). Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Frontiers in Microbiology, 6, 420.
Kumar, L., Chhibber, S., Kumar, R., Kumar, M., & Harjai, K. (2015). Zingerone silences quorum sensing and attenuates virulence of Pseudomonas aeruginosa. Fitoterapia, 102, 84–95.
Luo, J., Dong, B., Wang, K., Cai, S., Liu, T., Cheng, X., Lei, D., Chen, Y., Li, Y., & Kong, J. (2017). Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE, 12, e0176883.
Li, Y., Huang, J., Li, L., & Liu, L. (2017). Synergistic activity of berberine with azithromycin against Pseudomonas aeruginosa isolated from patients with cystic fibrosis of lung in vitro and in vivo. Cellular Physiology and Biochemistry, 42, 1657–1669.
Vasavi, H. S., Arun, A. B., & Rekha, P. D. (2014). Anti-quorum sensing activity of Psidium guajava L. flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1. Microbiology and Immunology, 58, 286–293.
Jakobsen, T. H., Warming, A. N., Vejborg, R. M., Moscoso, J. A., Stegger, M., Lorenzen, F., Rybtke, M., Andersen, J. B., Petersen, R., & Andersen, P. S. (2017). A broad range quorum sensing inhibitor working through sRNA inhibition. Science and Reports, 7, 1–12.
Ilic-Tomić, T., Soković, M., Vojnović, S., Ćirić, A. D., Veljić, M., Nikodinović-Runić, J., & Novaković, M. M. (2017). Diarylheptanoids from Alnus viridis ssp viridis and Alnus glutinosa: Modulation of quorum sensing activity in Pseudomonas aeruginosa. Planta Medica, 83, 117–125.
Nafee, N., Husari, A., Maurer, C. K., Lu, C., de Rossi, C., Steinbach, A., Hartmann, R. W., Lehr, C.-M., & Schneider, M. (2014). Antibiotic-free nanotherapeutics: Ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. Journal of Controlled Release, 192, 131–140.
Geske, G. D., Wezeman, R. J., Siegel, A. P., & Blackwell, H. E. (2005). Small molecule inhibitors of bacterial quorum sensing and biofilm formation. Journal of the American Chemical Society, 127, 12762–12763.
Hentzer, M., Riedel, K., Rasmussen, T. B., Heydorn, A., Andersen, J. B., Parsek, M. R., Rice, S. A., Eberl, L., Molin, S., & Høiby, N. (2002). Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology, 148, 87–102.
Lönn-Stensrud, J., Petersen, F., Benneche, T., & Scheie, A. A. (2007). Synthetic bromated furanone inhibits autoinducer-2-mediated communication and biofilm formation in oral streptococci. Oral Microbiology and Immunology, 22, 340–346.
O’Loughlin, C. T., Miller, L. C., Siryaporn, A., Drescher, K., Semmelhack, M. F., & Bassler, B. L. (2013). A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proceedings of the National Academy of Sciences, 110, 17981–17986.
Ishii, S., Fukui, K., Yokoshima, S., Kumagai, K., Beniyama, Y., Kodama, T., Fukuyama, T., Okabe, T., Nagano, T., & Kojima, H. (2017). High-throughput screening of small molecule inhibitors of the Streptococcus quorum-sensing signal pathway. Science and Reports, 7, 1–10.
Heidari, A., Noshiranzadeh, N., Haghi, F., & Bikas, R. (2017). Inhibition of quorum sensing related virulence factors of Pseudomonas aeruginosa by pyridoxal lactohydrazone. Microbial Pathogenesis, 112, 103–110.
Heidari, A., Haghi, F., Noshiranzadeh, N., & Bikas, R. (2017). (S, E)-2-hydroxy-N-(2-hydroxy-5-nitrobenzylidene) propane hydrazide as a quorum sensing inhibitor of Pseudomonas aeruginosa. Medicinal Chemistry Research, 26, 1947–1955.
Alizadeh, N., Memar, M., Mehramuz, B., Abibiglou, S., Hemmati, F., & Samadi Kafil, H. (2018). Current advances in aptamer-assisted technologies for detecting bacterial and fungal toxins. Journal of Applied Microbiology, 124, 644–651.
Ning, Y., Cheng, L., Ling, M., Feng, X., Chen, L., Wu, M., & Deng, L. (2015). Efficient suppression of biofilm formation by a nucleic acid aptamer. Pathogens and Disease, 73, ftv034.
Nimjee, S. M., Rusconi, C. P., & Sullenger, B. A. (2005). Aptamers: An emerging class of therapeutics. Annual Review of Medicine, 56, 555–583.
Shatila, F., Yaşa, İ, & Yalçın, H. T. (2020). Inhibition of Salmonella enteritidis biofilms by Salmonella invasion protein-targeting aptamer. Biotechnology Letters. https://doi.org/10.1007/s10529-020-02920-2
Kolovskaya, O. S., Savitskaya, A. G., Zamay, T. N., Reshetneva, I. T., Zamay, G. S., Erkaev, E. N., Wang, X., Wehbe, M., Salmina, A. B., & Perianova, O. V. (2013). Development of bacteriostatic DNA aptamers for salmonella. Journal of Medicinal Chemistry, 56, 1564–1572.
Mao, B., Cheng, L., Wang, S., Zhou, J., & Deng, L. (2018). Combat biofilm by bacteriostatic aptamer-functionalized graphene oxide. Biotechnology and Applied Biochemistry, 65, 355–361.
Lijuan, C., **ng, Y., Minxi, W., Wenkai, L., & Le, D. (2017). Development of an aptamer-ampicillin conjugate for treating biofilms. Biochemical and Biophysical Research Communications, 483, 847–854.
Thevendran, R., Sarah, S., Tang, T.-H., & Citartan, M. (2020). Strategies to bioengineer aptamer-driven nanovehicles as exceptional molecular tools for targeted therapeutics: A review. Journal of Controlled Release. https://doi.org/10.1016/j.jconrel.2020.04.051
Yu, Y. M., Xu, B. Y., Yan, S. S., Xu, J. F., Liu, F., Li, G. M., Ding, Y. L., & Wu, S. Q. (2013). Screening and anti-virulent study of N-acyl homoserine lactones DNA aptamers against Pseudomonas aeruginosa quorum sensing. Biotechnology and Bioprocess Engineering, 18, 406–412.
Wang, S., Mao, B., Wu, M., Liang, J., & Deng, L. (2018). Influence of aptamer-targeted antibiofilm agents for treatment of Pseudomonas aeruginosa biofilms. Antonie van Leeuwenhoek, 111, 199–208.
Oroh, S. B., Mustopa, A. Z., Budiarti, S., & Budiarto, B. R. (2020). Inhibition of enteropathogenic Escherichia coli biofilm formation by DNA aptamer. Molecular Biology Reports. https://doi.org/10.1007/s11033-020-05822-8
Sengupta, B., Adhikari, P., Mallet, E., Havner, R., & Pradhan, P. (2020). Spectroscopic study on Pseudomonas aeruginosa biofilm in the presence of the Aptamer-DNA scaffolded silver nanoclusters. Molecules, 25, 3631.
Whitesides, G. M. (2005). Nanoscience, nanotechnology, and chemistry. Small (Weinheim an der Bergstrasse, Germany), 1, 172–179.
Chen, M., Yu, Q., & Sun, H. (2013). Novel strategies for the prevention and treatment of biofilm related infections. International Journal of Molecular Sciences, 14, 18488–18501.
Mahamuni-Badiger, P. P., Patil, P. M., Badiger, M. V., Patel, P. R., Thorat-Gadgil, B. S., Pandit, A., & Bohara, R. A. (2019). Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Materials Science and Engineering: C, 108, 110319.
Shi, S.-F., Jia, J.-F., Guo, X.-K., Zhao, Y.-P., Chen, D.-S., Guo, Y.-Y., & Zhang, X.-L. (2016). Reduced Staphylococcus aureus biofilm formation in the presence of chitosan-coated iron oxide nanoparticles. International JOURNAL of Nanomedicine, 11, 6499.
Gupta, D., Singh, A., & Khan, A. U. (2017). Nanoparticles as efflux pump and biofilm inhibitor to rejuvenate bactericidal effect of conventional antibiotics. Nanoscale Research Letters, 12, 1–6.
Padwal, P., Bandyopadhyaya, R., & Mehra, S. (2014). Polyacrylic acid-coated iron oxide nanoparticles for targeting drug resistance in mycobacteria. Langmuir, 30, 15266–15276.
Banoee, M., Seif, S., Nazari, Z. E., Jafari-Fesharaki, P., Shahverdi, H. R., Moballegh, A., Moghaddam, K. M., & Shahverdi, A. R. (2010). ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 93, 557–561.
Nallathamby, P. D., Lee, K. J., Desai, T., & Xu, X.-H.N. (2010). Study of the multidrug membrane transporter of single living Pseudomonas aeruginosa cells using size-dependent plasmonic nanoparticle optical probes. Biochemistry, 49, 5942–5953.
Pérez-Laguna, V., García-Luque, I., Ballesta, S., Pérez-Artiaga, L., Lampaya-Pérez, V., Samper, S., Soria-Lozano, P., Rezusta, A., & Gilaberte, Y. (2018). Antimicrobial photodynamic activity of Rose Bengal, alone or in combination with Gentamicin, against planktonic and biofilm Staphylococcus aureus. Photodiagnosis and Photodynamic Therapy, 21, 211–216.
Khan, F., Lee, J.-W., Manivasagan, P., Pham, D. T. N., Oh, J., & Kim, Y.-M. (2019). Synthesis and characterization of chitosan oligosaccharide-capped gold nanoparticles as an effective antibiofilm drug against the Pseudomonas aeruginosa PAO1. Microbial Pathogenesis, 135, 103623.
Wang, Z., Bai, H., Lu, C., Hou, C., Qiu, Y., Zhang, P., Duan, J., & Mu, H. (2019). Light controllable chitosan micelles with ROS generation and essential oil release for the treatment of bacterial biofilm. Carbohydrate Polymers, 205, 533–539.
Kavanaugh, J. S., Flack, C. E., Lister, J., Ricker, E. B., Ibberson, C. B., Jenul, C., Moormeier, D. E., Delmain, E. A., Bayles, K. W., & Horswill, A. R. (2019). Identification of extracellular DNA-binding proteins in the biofilm matrix. MBio, 10, e01137–e1119.
**a, T., Kovochich, M., Liong, M., Madler, L., Gilbert, B., Shi, H., Yeh, J. I., Zink, J. I., & Nel, A. E. (2008). Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano, 2, 2121–2134.
Zhang, L., Jiang, Y., Ding, Y., Povey, M., & York, D. (2007). Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). Journal of Nanoparticle Research, 9, 479–489.
Ebrahimzadeh, S., Bari, M. R., Hamishehkar, H., Kafil, H. S., & Lim, L.-T. (2021). Essential oils-loaded electrospun chitosan-poly(vinyl alcohol) nonwovens laminated on chitosan film as bilayer bioactive edible films. LWT, 144, 111217.
Ma, L., Conover, M., Lu, H., Parsek, M. R., Bayles, K., & Wozniak, D. J. (2009). Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathogens, 5, e1000354.
Whitchurch, C. B., Tolker-Nielsen, T., Ragas, P. C., & Mattick, J. S. (2002). Extracellular DNA required for bacterial biofilm formation. Science, 295, 1487–1487.
Kovach, K., Fleming, D., Rumbaugh, K. P., & Gordon, V. (2019). Specific disruption of established P. aeruginosa biofilms using polymer-attacking enzymes. bioRxiv, 598979.
Zhang, L., Jiang, Y., Ding, Y., Daskalakis, N., Jeuken, L., Povey, M., O’Neill, A. J., & York, D. W. (2010). Mechanistic investigation into antibacterial behaviour of suspensions of ZnO nanoparticles against E. coli. Journal of Nanoparticle Research, 12, 1625–1636.
Messiaen, A.-S., Forier, K., Nelis, H., Braeckmans, K., & Coenye, T. (2013). Transport of nanoparticles and tobramycin-loaded liposomes in Burkholderia cepacia complex biofilms. PLoS ONE, 8, e79220.
Pham, D. T. N., Khan, F., Phan, T. T. V., Park, S.-K., Manivasagan, P., Oh, J., & Kim, Y.-M. (2019). Biofilm inhibition, modulation of virulence and motility properties by FeOOH nanoparticle in Pseudomonas aeruginosa. Brazilian Journal of Microbiology, 50, 791–805.
Loo, C. Y., Rohanizadeh, R., Young, P. M., Traini, D., Cavaliere, R., Whitchurch, C. B., & Lee, W. H. (2016). Combination of silver nanoparticles and curcumin nanoparticles for enhanced anti-biofilm activities. Journal of Agriculture and Food Chemistry, 64, 2513–2522.
Ghotaslou, R., Bahari, Z., Aliloo, A., Gholizadeh, P., & Eshlaghi, B. S. (2017). The in vitro effects of silver nanoparticles on bacterial biofilms. Journal of Microbiology, Biotechnology and Food Sciences, 6, 1077–1080.
Mu, H., Tang, J., Liu, Q., Sun, C., Wang, T., & Duan, J. (2016). Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Science and Reports, 6, 1–9.
Lin, W.-T., Tan, H.-L., Duan, Z.-L., Yue, B., Ma, R., He, G., & Tang, T.-T. (2014). Inhibited bacterial biofilm formation and improved osteogenic activity on gentamicin-loaded titania nanotubes with various diameters. International Journal of Nanomedicine, 9, 1215.
Khan, F., Manivasagan, P., Lee, J.-W., Pham, D. T. N., Oh, J., & Kim, Y.-M. (2019). Fucoidan-stabilized gold nanoparticle-mediated biofilm inhibition, attenuation of virulence and motility properties in Pseudomonas aeruginosa PAO1. Marine Drugs, 17, 208.
Ali, S. G., Ansari, M. A., Khan, H. M., Jalal, M., Mahdi, A. A., & Cameotra, S. S. (2017). Crataeva nurvala nanoparticles inhibit virulence factors and biofilm formation in clinical isolates of Pseudomonas aeruginosa. Journal of Basic Microbiology, 57, 193–203.
Huang, J., Liu, Y., Yang, L., & Zhou, F. (2019). Synthesis of sulfonated chitosan and its antibiofilm formation activity against E. coli and S. aureus. International Journal of Biological Macromolecules, 129, 980–988.
Khan, S. T., Ahmad, J., Ahamed, M., Musarrat, J., & Al-Khedhairy, A. A. (2016). Zinc oxide and titanium dioxide nanoparticles induce oxidative stress, inhibit growth, and attenuate biofilm formation activity of Streptococcus mitis. JBIC Journal of Biological Inorganic Chemistry, 21, 295–303.
Li, X., Wong, C.-H., Ng, T.-W., Zhang, C.-F., Leung, K.C.-F., & **, L. (2016). The spherical nanoparticle-encapsulated chlorhexidine enhances anti-biofilm efficiency through an effective releasing mode and close microbial interactions. International Journal of Nanomedicine, 11, 2471.
Wojciechowska, M., Równicki, M., Mieczkowski, A., Miszkiewicz, J., & Trylska, J. (2020). Antibacterial peptide nucleic acids—Facts and perspectives. Molecules, 25, 559.
Lee, H. T., Kim, S. K., & Yoon, J. W. (2019). Antisense peptide nucleic acids as a potential anti-infective agent. Journal of Microbiology, 57, 423–430.
Kurupati, P., Tan, K. S. W., Kumarasinghe, G., & Poh, C. L. (2007). Inhibition of gene expression and growth by antisense peptide nucleic acids in a multiresistant β-lactamase-producing Klebsiella pneumoniae strain. Antimicrobial Agents and Chemotherapy, 51, 805–811.
Barkowsky, G., Lemster, A.-L., Pappesch, R., Jacob, A., Krüger, S., Schröder, A., Kreikemeyer, B., & Patenge, N. (2019). Influence of different cell-penetrating peptides on the antimicrobial efficiency of PNAs in Streptococcus pyogenes. Molecular Therapy-Nucleic Acids, 18, 444–454.
Narenji, H., Gholizadeh, P., Aghazadeh, M., Rezaee, M. A., Asgharzadeh, M., & Kafil, H. S. (2017). Peptide nucleic acids (PNAs): Currently potential bactericidal agents. Biomedicine & Pharmacotherapy, 93, 580–588.
Readman, J. B., Dickson, G., & Coldham, N. G. (2017). Tetrahedral DNA nanoparticle vector for intracellular delivery of targeted peptide nucleic acid antisense agents to restore antibiotic sensitivity in cefotaxime-resistant Escherichia coli. Nucleic Acid Therapeutics, 27, 176–181.
Otsuka, T., Brauer, A. L., Kirkham, C., Sully, E. K., Pettigrew, M. M., Kong, Y., Geller, B. L., & Murphy, T. F. (2016). Antimicrobial activity of antisense peptide–peptide nucleic acid conjugates against non-typeable Haemophilus influenzae in planktonic and biofilm forms. Journal of Antimicrobial Chemotherapy, 72, 137–144.
Doyle, T. B., Hawkins, A. C., & McCarter, L. L. (2004). The complex flagellar torque generator of Pseudomonas aeruginosa. Journal of Bacteriology, 186, 6341–6350.
**a, Y., **ong, Y., Li, X., & Su, X. (2011). Inhibition of biofilm formation by the antisense peptide nucleic acids targeted at the motA gene in Pseudomonas aeruginosa PAO1 strain. World Journal of Microbiology & Biotechnology, 27, 1981–1987.
Castillo, J. I., Równicki, M., Wojciechowska, M., & Trylska, J. (2018). Antimicrobial synergy between mRNA targeted peptide nucleic acid and antibiotics in E. coli. Bioorganic & Medicinal Chemistry Letters, 28, 3094–3098.
Author information
Authors and Affiliations
Contributions
FH: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Roles/Writing—original draft; Writing—review & editing. MAR, SE, LY, RN & HSK: Data curation; Roles/Writing—original draft; Writing—review & editing. PG: Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hemmati, F., Rezaee, M.A., Ebrahimzadeh, S. et al. Novel Strategies to Combat Bacterial Biofilms. Mol Biotechnol 63, 569–586 (2021). https://doi.org/10.1007/s12033-021-00325-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00325-8