Abstract
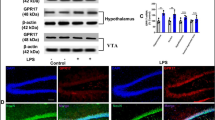
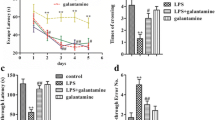
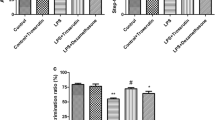
Our previous research found that activation of GPR55 can alleviate cognitive impairment induced by amyloid-beta 1–42 (Aβ1-42) and streptozotocin in mice, but the role of GPR55 in the pathogenesis of cognitive impairment remains unknown. Here, we used a lipopolysaccharide (LPS) mouse model to further investigate the role and mechanism of O-1602, a GPR55 agonist, on cognitive dysfunction. ICR mice were treated with an intracerebroventricular (i.c.v.) injection of LPS, followed by cognitive function tests. The expression of GPR55, NF-κB p65, caspase-3, Bax, and Bcl-2 in the hippocampus was examined by Western blotting. Inflammatory cytokines and microglia were detected by ELISA kit and immunohistochemical analyses, respectively. The levels of MDA, GSH, SOD, and CAT were examined by assay kits. Furthermore, TUNEL-staining was used to detect neuronal apoptosis. Our results showed that i.c.v. injection of LPS in mice exhibited impaired performance in the behavior tests, which were ameliorated by O-1602 treatment (2.0 or 4.0 μg/mouse, i.c.v.). Importantly, we found that O-1602 treatment reversed GPR55 downregulation, decreased the expression of NF-κB p65, suppressed the accumulation of proinflammatory cytokines and microglia activation, increased the anti-inflammatory cytokines, and reduced the levels of MDA, increased the levels of GSH, SOD, and CAT in the hippocampus. In addition, O-1602 treatment also significantly reduced Bax and increased Bcl-2 expression as well as decreased caspase-3 activity and TUNEL-positive cells in the hippocampus. These observations indicate that O-1602 may ameliorate LPS-induced cognition deficits via inhibiting neuroinflammation, oxidative stress, and apoptosis mediated by the NF-κB pathway in mice.










Similar content being viewed by others
Data Availability
We declared that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
References
Baik SH, Kang S, Lee W, Choi H, Chung S, Kim JI, Mook-Jung I (2019) A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell Metab 30:493–507
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Bussi C, Peralta Ramos JM, Arroyo DS, Gaviglio EA, Gallea JI, Wang JM, Celej MS, Iribarren P (2017) Autophagy down regulates pro-inflammatory mediators in BV2 microglial cells and rescues both LPS and alpha-synuclein induced neuronal cell death. Sci Rep 7:43153
Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20:148–160
Celorrio M, Rojo-Bustamante E, Fernandez-Suarez D, Saez E, Estella-Hermoso de Mendoza A, Muller CE, Ramirez MJ, Oyarzabal J, Franco R, Aymerich MS (2017) GPR55: a therapeutic target for Parkinson’s disease? Neuropharmacology 125:319–332
Clark RS, Kochanek PM, Watkins SC, Chen M, Dixon CE, Seidberg NA, Melick J, Loeffert JE, Nathaniel PD, ** KL, Graham SH (2000) Caspase-3 mediated neuronal death after traumatic brain injury in rats. J Neurochem 74:740–753
D’Amelio M, Cavallucci V, Cecconi F (2010) Neuronal caspase-3 signaling: not only cell death. Cell Death Differ 17:1104–1114
Deliu E, Sperow M, Console-Bram L, Carter RL, Tilley DG, Kalamarides DJ, Kirby LG, Brailoiu GC, Brailoiu E, Benamar K, Abood ME (2015) The lysophosphatidylinositol receptor GPR55 modulates pain perception in the periaqueductal gray. Mol Pharmacol 88:265–272
Fourrier C, Remus-Borel J, Greenhalgh AD, Guichardant M, Bernoud-Hubac N, Lagarde M, Joffre C, Laye S (2017) Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J Neuroinflammation 14:170
Fruhauf PK, Ineu RP, Tomazi L, Duarte T, Mello CF, Rubin MA (2015) Spermine reverses lipopolysaccharide-induced memory deficit in mice. J Neuroinflammation 12:3
Fu L, Liu C, Chen L, Lv Y, Meng G, Hu M, Long Y, Hong H, Tang S (2019) Protective effects of 1-methylnicotinamide on Abeta1-42-induced cognitive deficits, neuroinflammation and apoptosis in mice. J Neuroimmune Pharmacol 14:401–412
Gandhi S, Abramov AY (2012) Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev 2012:428010
Gao J, **ong B, Zhang B, Li S, Huang N, Zhan G, Jiang R, Yang L, Wu Y, Miao L, Zhu B, Yang C, Luo A (2018) Sulforaphane alleviates lipopolysaccharide-induced spatial learning and memory dysfunction in mice: the role of BDNF-mTOR signaling pathway. Neuroscience 388:357–366
Goel R, Bhat SA, Hanif K, Nath C, Shukla R (2018) Angiotensin II receptor blockers attenuate lipopolysaccharide-induced memory impairment by modulation of NF-kappaB-mediated BDNF/CREB expression and apoptosis in spontaneously hypertensive rats. Mol Neurobiol 55:1725–1739
Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16:358–372
Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148:1204–1222
Hurst K, Badgley C, Ellsworth T, Bell S, Friend L, Prince B, Welch J, Cowan Z, Williamson R, Lyon C, Anderson B, Poole B, Christensen M, McNeil M, Call J, Edwards JG (2017) A putative lysophosphatidylinositol receptor GPR55 modulates hippocampal synaptic plasticity. Hippocampus 27:985–998
Janelidze S, Mattsson N, Stomrud E, Lindberg O, Palmqvist S, Zetterberg H, Blennow K, Hansson O (2018) CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology 91:e867–e877
Khan MS, Ali T, Kim MW, Jo MH, Jo MG, Badshah H, Kim MO (2016) Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem Int 100:1–10
Lee HJ, Lim SM, Ko DB, Jeong JJ, Hwang YH, Kim DH (2017) Soyasapogenol B and genistein attenuate lipopolysaccharide-induced memory impairment in mice by the modulation of NF-kappaB-mediated BDNF expression. J Agric Food Chem 65:6877–6885
Lee YJ, Choi DY, Yun YP, Han SB, Oh KW, Hong JT (2013) Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J Nutr Biochem 24:298–310
Lei S, Wu S, Wang G, Li B, Liu B, Lei X (2021) Pinoresinol diglucoside attenuates neuroinflammation, apoptosis and oxidative stress in a mice model with Alzheimer’s disease. NeuroReport 32:259–267
Lipina C, Walsh SK, Mitchell SE, Speakman JR, Wainwright CL, Hundal HS (2019) GPR55 deficiency is associated with increased adiposity and impaired insulin signaling in peripheral metabolic tissues. FASEB J 33:1299–1312
Liu Y, Zhang Y, Zheng X, Fang T, Yang X, Luo X, Guo A, Newell KA, Huang XF, Yu Y (2018) Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J Neuroinflammation 15:112
Martini F, Rosa SG, Klann IP, Fulco BCW, Carvalho FB, Rahmeier FL, Fernandes MC, Nogueira CW (2019) A multifunctional compound ebselen reverses memory impairment, apoptosis and oxidative stress in a mouse model of sporadic Alzheimer’s disease. J Psychiatr Res 109:107–117
Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T (2007) Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun 362:928–934
Pintado C, Gavilan MP, Gavilan E, Garcia-Cuervo L, Gutierrez A, Vitorica J, Castano A, Rios RM, Ruano D (2012) Lipopolysaccharide-induced neuroinflammation leads to the accumulation of ubiquitinated proteins and increases susceptibility to neurodegeneration induced by proteasome inhibition in rat hippocampus. J Neuroinflammation 9:87
Rahimi A, Hajizadeh Moghaddam A, Roohbakhsh A (2015) Central administration of GPR55 receptor agonist and antagonist modulates anxiety-related behaviors in rats. Fundam Clin Pharmacol 29:185–190
Reid SNS, Ryu JK, Kim Y, Jeon BH (2018) GABA-enriched fermented Laminaria japonica improves cognitive impairment and neuroplasticity in scopolamine- and ethanol-induced dementia model mice. Nurs Res Pract 12:199–207
Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ (2007) The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152:1092–1101
Saliba SW, Jauch H, Gargouri B, Keil A, Hurrle T, Volz N, Mohr F, van der Stelt M, Brase S, Fiebich BL (2018) Anti-neuroinflammatory effects of GPR55 antagonists in LPS-activated primary microglial cells. J Neuroinflammation 15:322
Sylantyev S, Jensen TP, Ross RA, Rusakov DA (2013) Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci USA 110:5193–5198
Tang J, Diao P, Shu X, Li L, **ong L (2019) Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-Induced RAW264.7 cells: in vitro assessment and a theoretical model. BioMed Res Int 2019:7039802
Tonnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimer’s Dis 57:1105–1121
Tripathi A, Paliwal P, Krishnamurthy S (2017) Piracetam attenuates LPS-induced neuroinflammation and cognitive impairment in rats. Cell Mol Neurobiol 37:1373–1386
Unger MS, Schernthaner P, Marschallinger J, Mrowetz H, Aigner L (2018) Microglia prevent peripheral immune cell invasion and promote an anti-inflammatory environment in the brain of APP-PS1 transgenic mice. J Neuroinflammation 15:274
Vong CT, Tseng HHL, Kwan YW, Lee SM, Hoi MPM (2019) Novel protective effect of O-1602 and abnormal cannabidiol, GPR55 agonists, on ER stress-induced apoptosis in pancreatic beta-cells. Biomed Pharmacother 111:1176–1186
Wang LS, Tao X, Liu XM, Zhou YF, Zhang MD, Liao YH, Pan RL, Chang Q (2019) Cajaninstilbene acid ameliorates cognitive impairment induced by intrahippocampal injection of amyloid-beta1-42 oligomers. Front Pharmacol 10:1084
Wang Y, Gu J, Hu L, Kong L, Wang T, Di M, Li C, Gui S (2020) miR-130a alleviates neuronal apoptosis and changes in expression of Bcl-2/Bax and caspase-3 in cerebral infarction rats through PTEN/PI3K/Akt signaling pathway. Exp Ther Med 19:2119–2126
Wang YW, Zhou Q, Zhang X, Qian QQ, Xu JW, Ni PF, Qian YN (2017) Mild endoplasmic reticulum stress ameliorates lipopolysaccharide-induced neuroinflammation and cognitive impairment via regulation of microglial polarization. J Neuroinflammation 14:233
Wargent ET, Kepczynska M, Zaibi MS, Hislop DC, Arch JRS, Stocker CJ (2020) High fat-fed GPR55 null mice display impaired glucose tolerance without concomitant changes in energy balance or insulin sensitivity but are less responsive to the effects of the cannabinoids rimonabant or Delta(9)-tetrahydrocannabivarin on weight gain. PeerJ 8:e9811
Wrobel A, Serefko A, Szopa A, Ulrich D, Poleszak E, Rechberger T (2020) O-1602, an agonist of atypical cannabinoid receptors GPR55, reverses the symptoms of depression and detrusor overactivity in rats subjected to corticosterone treatment. Front Pharmacol 11:1002
Wu H, Wang X, Gao J, Liang S, Hao Y, Sun C, **a W, Cao Y, Wu L (2017) Fingolimod (FTY720) attenuates social deficits, learning and memory impairments, neuronal loss and neuroinflammation in the rat model of autism. Life Sci 173:43–54
Wu X, Lv YG, Du YF, Chen F, Reed MN, Hu M, Suppiramaniam V, Tang SS, Hong H (2018) Neuroprotective effects of INT-777 against Abeta1-42-induced cognitive impairment, neuroinflammation, apoptosis, and synaptic dysfunction in mice. Brain Behav Immun 73:533–545
Wu X, Lv YG, Du YF, Hu M, Reed MN, Long Y, Suppiramaniam V, Hong H, Tang SS (2019) Inhibitory effect of INT-777 on lipopolysaccharide-induced cognitive impairment, neuroinflammation, apoptosis, and synaptic dysfunction in mice. Prog Neuropsychopharmacol Biol Psychiatry 88:360–374
**ang X, Wang X, ** S, Hu J, Wu Y, Li Y, Wu X (2022a) Activation of GPR55 attenuates cognitive impairment and neurotoxicity in a mouse model of Alzheimer’s disease induced by Abeta1-42 through inhibiting RhoA/ROCK2 pathway. Prog Neuropsychopharmacol Biol Psychiatry 112:110423
**ang X, Wang X, Wu Y, Hu J, Li Y, ** S, Wu X (2022b) Activation of GPR55 attenuates cognitive impairment, oxidative stress, neuroinflammation, and synaptic dysfunction in a streptozotocin-induced Alzheimer’s mouse model. Pharmacol Biochem Behav 214:173340
You LH, Yan CZ, Zheng BJ, Ci YZ, Chang SY, Yu P, Gao GF, Li HY, Dong TY, Chang YZ (2017) Astrocyte hepcidin is a key factor in LPS-induced neuronal apoptosis. Cell Death Dis 8:e2676
Zhang XY, Xu ZP, Wang W, Cao JB, Fu Q, Zhao WX, Li Y, Huo XL, Zhang LM, Li YF, Mi WD (2018) Vitamin C alleviates LPS-induced cognitive impairment in mice by suppressing neuroinflammation and oxidative stress. Int Immunopharmacol 65:438–447
Zhao WX, Zhang JH, Cao JB, Wang W, Wang DX, Zhang XY, Yu J, Zhang YY, Zhang YZ, Mi WD (2017) Acetaminophen attenuates lipopolysaccharide-induced cognitive impairment through antioxidant activity. J Neuroinflammation 14:17
Zhao Y, Deng H, Li K, Wang L, Wu Y, Dong X, Wang X, Chen Y, Xu Y (2019) Trans-cinnamaldehyde improves neuroinflammation-mediated NMDA receptor dysfunction and memory deficits through blocking NF-kappaB pathway in presenilin1/2 conditional double knockout mice. Brain Behav Immun 82:45–62
Zhu X, Liu J, Chen S, Xue J, Huang S, Wang Y, Chen O (2019) Isoliquiritigenin attenuates lipopolysaccharide-induced cognitive impairment through antioxidant and anti-inflammatory activity. BMC Neurosci 20:41
Funding
This project was supported by the Natural Science Foundation of Anhui Province (2108085QH385), Doctor Foundation of Anhui Medical University (0601086201), Anhui Medical University School Funding Project (2020xkj012), and National undergraduate innovation and entrepreneurship training program (202010366022).
Author information
Authors and Affiliations
Contributions
XW conceived and designed the study. XTX, JH, YMW, YYL, and SYJ performed the experiments and collected the data. XW analyzed the data, and drafted this article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The studies involving animals were approved and performed in accordance with the ethical standards of the institutional animal ethics committee in Anhui Medical University.
Consent for Publication
All authors have seen the manuscript and given their consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
**n Wang, **aoTong **ang, and Jie Hu contributed equally to this work.
Highlights
• Activation of GPR55 ameliorates cognitive impairment induced by LPS in mice.
• Activation of GPR55 protects against LPS-induced neuroinflammation, oxidative stress, and apoptosis via inhibition of the NF-κB pathway in mice.
• The central GPR55 may play an important role in cognitive dysfunction.
Rights and permissions
About this article
Cite this article
Wang, X., **ang, X., Hu, J. et al. Pharmacological Activation of GPR55 Improved Cognitive Impairment Induced by Lipopolysaccharide in Mice. J Mol Neurosci 72, 1656–1669 (2022). https://doi.org/10.1007/s12031-022-02020-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-022-02020-y