Abstract
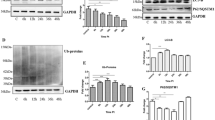
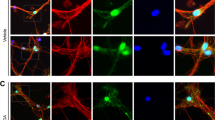
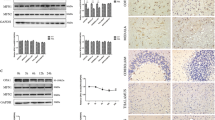
Axonal degeneration is a hallmark of many neurodegenerative disorders including transmissible spongiform encephalopathies (TSE). However, the full complement of axonal degeneration triggers is not fully understood. In an in vitro prion model, we observed that treatment of rat spinal neurons with the prion peptide, PrP106-126, activated death receptor 6 (DR6, also known as TNFRSF21), caspase-6, caspase-3, and induced axonal degeneration. Knockdown of DR6 by siRNA blocked caspase-6 and caspase-3 activation and axonal degeneration. We also found that cleaved caspase-3 is only enriched in cell bodies, but cleaved caspase-6 is expressed in both cell bodies and axons. Axonal degeneration was prevented by preincubation of neurons with a caspase-6 inhibitor or siRNA of caspase-6. Our findings suggest that both DR6 and caspase-6 play important roles in axonal degeneration and caspase-6 acts downstream of DR6. We also observed that nicotinamide nucleotide adenylyltransferase 1 protein (Nmnat1), which had been reported to protect neurons from degeneration, alleviated axonal degeneration without blocking caspase-6 activation, suggesting that Nmnat acts downstream or parallel to caspase-6 activation. Our results indicate that PrP106-126 triggered axonal degeneration of the spinal cord neurons, DR6 is a key regulator of axonal degeneration, and the signaling pathway of DR6/caspase-6 mediates axonal degeneration induced by the prion fragment. Our findings raise the hope of targeting the DR6 as a potential therapeutic strategy in prion-related neurodegenerative diseases.





Similar content being viewed by others
References
Bossen C et al (2006) Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem 281(20):13964–13971
Buss RR, Sun W, Oppenheim RW (2006) Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci 29:1–35
Coleman MP, Perry VH (2002) Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci 25(10):532–537
Conde C, Caceres A (2009) Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 10(5):319–332
Cunningham C et al (2003) Synaptic changes characterize early behavioural signs in the ME7 model of murine prion disease. Eur J Neurosci 17(10):2147–2155
Cusack CL et al (2013) Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nat Commun 4:1876
Forloni G et al (1993) Neurotoxicity of a prion protein fragment. Nature 362(6420):543–546
Fuhrmann M et al (2007) Dendritic pathology in prion disease starts at the synaptic spine. J Neurosci 27(23):6224–6233
Gerdts J et al (2013) Sarm1-mediated axon degeneration requires both SAM and TIR interactions. J Neurosci 33(33):13569–13580
Gray BC et al (2009) Selective presynaptic degeneration in the synaptopathy associated with ME7-induced hippocampal pathology. Neurobiol Dis 35(1):63–74
Guo H et al (2004) Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease. Am J Pathol 165(2):523–531
Haase G et al (2008) Signaling by death receptors in the nervous system. Curr Opin Neurobiol 18(3):284–291
Henriques ST et al (2008) PrP(106–126) does not interact with membranes under physiological conditions. Biophys J 95(4):1877–1889
Horowitz PM et al (2004) Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer’s disease. J Neurosci 24(36):7895–7902
Hu Y et al (2013) A DR6/p75(NTR) complex is responsible for beta-amyloid-induced cortical neuron death. Cell Death Dis 4:e579
Huang G et al (2013) Death receptor 6 (DR6) antagonist antibody is neuroprotective in the mouse SOD1G93A model of amyotrophic lateral sclerosis. Cell Death Dis 4:e841
Jeffrey M et al (2000) Synapse loss associated with abnormal PrP precedes neuronal degeneration in the scrapie-infected murine hippocampus. Neuropathol Appl Neurobiol 26(1):41–54
Klaiman G et al (2008) Targets of caspase-6 activity in human neurons and Alzheimer disease. Mol Cell Proteomics 7(8):1541–1555
Li H et al (2001) Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington’s disease mice. J Neurosci 21(21):8473–8481
Luo L, O’Leary DD (2005) Axon retraction and degeneration in development and disease. Annu Rev Neurosci 28:127–156
Medana IM, Esiri MM (2003) Axonal damage: a key predictor of outcome in human CNS diseases. Brain 126(Pt 3):515–530
Mi S et al (2011) Death receptor 6 negatively regulates oligodendrocyte survival, maturation and myelination. Nat Med 17(7):816–821
Nikolaev A et al (2009) APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 457(7232):981–989
Novitskaya V et al (2007) Amyloid fibrils of mammalian prion protein induce axonal degeneration in NTERA2-derived terminally differentiated neurons. J Neurochem 102(2):398–407
Pan G et al (1998) Identification and functional characterization of DR6, a novel death domain-containing TNF receptor. FEBS Lett 431(3):351–356
Prusiner SB (1991) Molecular biology of prion diseases. Science 252(5012):1515–1522
Raff MC, Whitmore AV, Finn JT (2002) Axonal self-destruction and neurodegeneration. Science 296(5569):868–871
Ren Y, Zhao J, Feng J (2003) Parkin binds to alpha/beta tubulin and increases their ubiquitination and degradation. J Neurosci 23(8):3316–3324
Sagot Y et al (1995) Bcl-2 overexpression prevents motoneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. J Neurosci 15(11):7727–7733
Saxena S, Caroni P (2007) Mechanisms of axon degeneration: from development to disease. Prog Neurobiol 83(3):174–191
Schulze-Osthoff K et al (1998) Apoptosis signaling by death receptors. Eur J Biochem 254(3):439–459
Scott JR (1993) Scrapie pathogenesis. Br Med Bull 49(4):778–791
Selvaggini C et al (1993) Molecular characteristics of a protease-resistant, amyloidogenic and neurotoxic peptide homologous to residues 106-126 of the prion protein. Biochem Biophys Res Commun 194(3):1380–1386
Shi F et al (2012) The NALP3 inflammasome is involved in neurotoxic prion peptide-induced microglial activation. J Neuroinflammation 9:73
Simon DJ et al (2012) A caspase cascade regulating developmental axon degeneration. J Neurosci 32(49):17540–17553
Siskova Z et al (2009) Degenerating synaptic boutons in prion disease: microglia activation without synaptic strip**. Am J Pathol 175(4):1610–1621
Stokin GB et al (2005) Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 307(5713):1282–1288
Taimen P, Kallajoki M (2003) NuMA and nuclear lamins behave differently in Fas-mediated apoptosis. J Cell Sci 116(Pt 3):571–583
Uribe V et al (2012) Rescue from excitotoxicity and axonal degeneration accompanied by age-dependent behavioral and neuroanatomical alterations in caspase-6-deficient mice. Hum Mol Genet 21(9):1954–1967
Vohra BP et al (2010) Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. J Neurosci 30(41):13729–13738
Watts RJ, Hoopfer ED, Luo L (2003) Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron 38(6):871–885
Acknowledgments
This work was supported by the Natural Science Foundation of China (Project No. 31272532 and No. 31172293), the Ministry of Agriculture Program of China (No. 2013-S11 and No. 2014-S9), and the Program for Cheung Kong Scholars and Innovative Research Team in the University of China (No. IRT0866).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yunsheng Wang and Deming Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhao, D., Pan, B. et al. Death Receptor 6 and Caspase-6 Regulate Prion Peptide-Induced Axonal Degeneration in Rat Spinal Neurons. J Mol Neurosci 56, 966–976 (2015). https://doi.org/10.1007/s12031-015-0562-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0562-1