Abstract
Background
Aneurysmal subarachnoid hemorrhage (aSAH) leads to a robust systemic inflammatory response. We hypothesized that an early systemic glycolytic shift occurs after aSAH, resulting in a unique metabolic signature and affecting systemic inflammation.
Methods
Control patients and patients with aSAH were analyzed. Samples from patients with aSAH were collected within 24 h of aneurysmal rupture. Mass spectrometry–based metabolomics was performed to assess relative abundance of 16 metabolites involved in the tricarboxylic acid cycle, glycolysis, and pentose phosphate pathway. Principal component analysis was used to segregate control patients from patients with aSAH. Dendrograms were developed to depict correlations between metabolites and cytokines. Analytic models predicting functional outcomes were developed, and receiver operating curves were compared.
Results
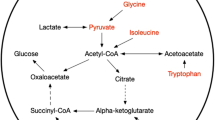
A total of 122 patients with aSAH and 38 control patients were included. Patients with aSAH had higher levels of glycolytic metabolites (3-phosphoglycerate/2-phosphoglycerate, lactate) but lower levels of oxidative metabolites (succinate, malate, fumarate, and oxalate). Patients with higher clinical severity (Hunt-Hess Scale score ≥ 4) had higher levels of glyceraldehyde 3-phosphate and citrate but lower levels of α-ketoglutarate and glutamine. Principal component analysis readily segregated control patients from patients with aSAH. Correlation analysis revealed distinct clusters in control patients that were not observed in patients with aSAH. Higher levels of fumarate were associated with good functional outcomes at discharge (odds ratio [OR] 1.76, 95% confidence interval [CI] 1.15–2.82) in multivariable models, whereas higher levels of citrate were associated with poor functional outcomes at discharge (OR 0.36, 95% CI 0.16–0.73) and at 3 months (OR 0.35, 95% CI 0.14–0.81). No associations were found with delayed cerebral ischemia. Levels of α-ketoglutarate and glutamine correlated with lower levels of interleukin-8, whereas fumarate was associated with lower levels of tumor necrosis factor alpha.
Conclusions
Aneurysmal subarachnoid hemorrhage results in a unique pattern of plasma metabolites, indicating a shift toward glycolysis. Higher levels of fumarate and lower levels of citrate were associated with better functional outcomes. These metabolites may represent targets to improve metabolism after aSAH.





Similar content being viewed by others
References
Connolly ES, Rabinstein A, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–37.
Chen S, Li Q, Wu H, Krafft PR, Wang Z, Zhang JH. The harmful effects of subarachnoid hemorrhage on extracerebral organs. Biomed Res Int. 2014;2014:858496.
Helbok R, Schmidt JM, Kurtz P, Hanafy K, Fernandez L, Stuart RM, et al. Systemic glucose and brain energy metabolism after subarachnoid hemorrhage. Neurocrit Care. 2010;12:317–23.
Kurtz P, Claassen J, Helbok R, Schmidt J, Fernandez L, Presciutti M, et al. Systemic glucose variability predicts cerebral metabolic distress and mortality after subarachnoid hemorrhage: a retrospective observational study. Crit Care. 2014;18:R89.
Dhar R, Diringer MN. The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit Care. 2008;8:404–12.
Savarraj J, Parsha K, Hergenroeder G, Ahn S, Chang TR, Kim DH, et al. Early brain injury associated with systemic inflammation after subarachnoid hemorrhage. Neurocrit Care. 2018;28:203–11.
Savarraj JPJ, Parsha K, Hergenroeder GW, Zhu L, Bajgur SS, Ahn S, et al. Systematic model of peripheral inflammation after subarachnoid hemorrhage. Neurology. 2017;88:1535–45.
Savarraj JP, McGuire MF, Parsha K, Hergenroeder G, Bajgur S, Ahn S, et al. Disruption of thrombo-inflammatory response and activation of a distinct cytokine cluster after subarachnoid hemorrhage. Cytokine. 2018;111:334–41.
Mills EL, Kelly B, O’Neill LAJ. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017;18:488–98.
O’Neill LAJ. A broken krebs cycle in macrophages. Immunity. 2015;42:393–4.
O’Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–65.
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59:21–7 (discussion 21–7).
Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5.
Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–6.
Amara CS, Ambati CR, Vantaku V, Badrajee Piyarathna DW, Donepudi SR, Ravi SS, et al. Serum metabolic profiling identified a distinct metabolic signature in bladder cancer smokers: a key metabolic enzyme associated with patient survival. Cancer Epidemiol Biomarkers Prev. 2019;28:770–81.
Johnson SC. Hierarchical clustering schemes. Psychometrika. 1967;32:241–54.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300.
Iacobazzi V, Infantino V. Citrate-new functions for an old metabolite. Biol Chem. 2014;395:387–99.
Wallace DC. Mitochondria and cancer: Warburg addressed. Cold Spring Harb Symp Quant Biol. 2005;70:363–74.
Pålsson-McDermott EM, O’Neill LAJ. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020;30:300–14. https://doi.org/10.1038/s41422-020-0291-z.
Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016. p. 457–70. Available from: http://linkinghub.elsevier.com/retrieve/pii/S009286741631162X
Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–5.
Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol. 2009;86:174–9.
Tannahill GM, Curtis AM, Adamik J, Palsson-Mcdermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–42.
Watanabe K, Nagao M, Toh R, Irino Y, Shinohara M, Iino T, et al. Critical role of glutamine metabolism in cardiomyocytes under oxidative stress. Biochem Biophys Res Commun. 2021;534:687–93.
Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619–34.
Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–64.
Gao C, Liu X, Shi H, Xu S, Ji Z, Wang C, et al. Relationship between sympathetic nervous activity and inflammatory response after subarachnoid hemorrhage in a perforating canine model. Auton Neurosci. 2009;147:70–4.
Gaetani P, Tartara F, Pignatti P, Tancioni F, Rodriguez R, Baena B, De Benedetti F. Cisternal CSF levels of cytokines after subarachnoid hemorrhage. Neurol Res. 1998;20:337–42.
Beutler B. TNF, immunity and inflammatory disease: lessons of the past decade. J Investig Med. 1995;43:227–35.
Brasier AR, Jamaluddin M, Casola A, Duan W, Shen Q, Garofalo RP. A promoter recruitment mechanism for tumor necrosis factor-alpha-induced interleukin-8 transcription in type II pulmonary epithelial cells. Dependence on nuclear abundance of Rel A, NF-kappaB1, and c-Rel transcription factors. J Biol Chem. 1998;273:3551–61.
Chou SH-Y, Feske SK, Atherton J, Konigsberg RG, De Jager PL, Du R, et al. Early elevation of serum tumor necrosis factor-α is associated with poor outcome in subarachnoid hemorrhage. J Investig Med. 2012;60:1054–8.
He L, Li H, Huang N, Zhou X, Tian J, Li T, et al. Alpha-ketoglutarate suppresses the NF-κB-mediated inflammatory pathway and enhances the PXR-regulated detoxification pathway. Oncotarget. 2017;8:102974–88.
Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510:397–401.
Asadi Shahmirzadi A, Edgar D, Liao C-Y, Hsu Y-M, Lucanic M, Asadi Shahmirzadi A, et al. Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab. 2020;32:447–456.e6.
Hares P, James IM, Pearson RM. Effect of ornithine alpha ketoglutarate (OAKG) on the response of brain metabolism to hypoxia in the dog. Stroke. 1978;9:222–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/644619
Huang J, Liu J, Chang G, Wang Y, Ma N, Roy AC, et al. Glutamine Supplementation Attenuates the Inflammation Caused by LPS-Induced Acute Lung Injury in Mice by Regulating the TLR4/MAPK Signaling Pathway. Inflammation. 2021; Available from: http://www.ncbi.nlm.nih.gov/pubmed/34160729
Bernier L-P, York EM, Kamyabi A, Choi HB, Weilinger NL, MacVicar BA. Microglial metabolic flexibility supports immune surveillance of the brain parenchyma. Nat Commun. 2020;11:1559.
Nibbering PH, Thio B, Zomerdijk TP, Bezemer AC, Beijersbergen RL, van Furth R. Effects of monomethylfumarate on human granulocytes. J Invest Dermatol. 1993;101:37–42.
Litjens NHR, Burggraaf J, van Strijen E, van Gulpen C, Mattie H, Schoemaker RC, et al. Pharmacokinetics of oral fumarates in healthy subjects. Br J Clin Pharmacol. 2004;58:429–32.
Linker RA, Lee D-H, Ryan S, van Dam AM, Conrad R, Bista P, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–92.
Gafson AR, Savva C, Thorne T, David M, Gomez-Romero M, Lewis MR, et al. Breaking the cycle: Reversal of flux in the tricarboxylic acid cycle by dimethyl fumarate. Neurol Neuroimmunol Neuroinflammation. 2019;6:e562.
Owjfard M, Bigdeli MR, Safari A, Haghani M, Namavar MR. Effect of dimethyl fumarate on the motor function and spatial arrangement of primary motor cortical neurons in the sub-acute phase of stroke in a rat model. J Stroke Cerebrovasc Dis. 2021;30:105630.
Lin R, Cai J, Kostuk EW, Rosenwasser R, Iacovitti L. Fumarate modulates the immune/inflammatory response and rescues nerve cells and neurological function after stroke in rats. J Neuroinflammation. 2016;13:269.
Hou X, Xu H, Chen W, Zhang N, Zhao Z, Fang X, et al. Neuroprotective effect of dimethyl fumarate on cognitive impairment induced by ischemic stroke. Ann Transl Med. 2020;8:375.
Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55.
Patterson J, Shi X, Bresette W, Eghlimi R, Atlas S, Farr K, et al. A Metabolomic Analysis of the Sex-Dependent Hispanic Paradox. Metabolites. 2021;11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34436492
Acknowledgements
The authors acknowledge all patients who participated in this study.
Funding
This study was supported by intramural funding awarded to AMG by University of Texas Health Neurosciences. Funding for metabolomics experiments were partially supported by a grant awarded to NP for the operation of a metabolomics shared resource (National Cancer Institute, 5P30CA125123).
Author information
Authors and Affiliations
Contributions
AMG designed the study, analyzed data, and wrote the article. CF analyzed data and edited the article. VP acquitted and analyzed data and edited the article. ASP acquired data. HC acquired data. XR analyzed data and revised the article. MKH acquired data and edited the article. PD contributed to experimental design and revised the article. CC contributed to experimental design and analyzed data. NP contributed to experimental design, acquired data, and analyzed data. HAC contributed to experimental design and edited the article. JPS helped to design the study, analyzed data, and wrote and revised the article. The final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Gusdon reports an intramural grant from University of Texas Health Neurosciences during the conduct of the study. Dr. Coarfa reports grants from the National Cancer Institute (NCI), National Institute of Environmental Health Sciences (NIEHS), National Institute on Minority Health and Health Disparities (NIMHD), and Cancer Prevention and Research Institute of Texas (CPRIT) during the conduct of the study. Dr. N. Putluri reports grants from the NCI during the conduct of the study. The remaining authors have no conflicts to disclose.
Ethical approval/informed consent
This manuscript adheres to all ethical guidelines and was approved by the University of Texas McGovern School of Medicine Institutional Review Board (HSC-MS-12–0637).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gusdon, A.M., Fu, C., Putluri, V. et al. Early Systemic Glycolytic Shift After Aneurysmal Subarachnoid Hemorrhage is Associated with Functional Outcomes. Neurocrit Care 37, 724–734 (2022). https://doi.org/10.1007/s12028-022-01546-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01546-8