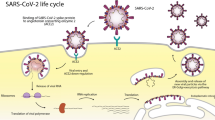
Abstract
Thrombotic events associated with SARS-CoV-2 at the vascular endothelium still remains unclear. The aim of the current study is to determine the relationship between cellular proteins on the (ocular) vascular endothelial surface and the immune thrombotic and/or endotheliopathy process elicited by SARS-CoV-2 using an in-silico modeling. The structural S (spike glycoprotein), N (nucleocapsid protein), M (membrane protein), and E (envelope protein) proteins, an accessory protein (ORF1ab) of SARS-CoV-2 and 158 cellular proteins associated with retinal vascular endothelial cell surface or structure were included in this study for comparison of three-dimensional (3D) structure and sequence. Sixty-nine of the retinal proteins were obtained from the Uniprot database. Remaining proteins not included in the database were included in the study after they were converted into 3D structures using the RaptorX web tool. Sequence and three-dimensional structure of SARS-COV-2 S, N, M, E, ORF1ab proteins and retinal vascular endothelial proteins were compared with mTM-align server. Proteins with significant similarity (score above 0.5) were validated with the TM-align web server. Immune and thrombosis-related protein-receptor interactions of similar proteins was checked with CABS-dock. We detected a high level of structural similarity between E protein and ACE, ACE2, LAT1, and TM9SF4 endothelial proteins. In addition, PECAM-1 was found to be structurally similar to ORF1ab and S protein. When we evaluated the likelihood/potential to stimulate an immune responses/a cytokine release, TLR-2 and TLR-3, which are highly susceptible to SARS-CoV2, showed a potential receptor-protein interaction with retinal vascular endothelial proteins. Our study demonstrates that SARS-CoV-2 proteins may have structural similarities with vascular endothelial proteins, and therefore, as immunological target sites, the counterpart proteins on the endothelial surface of many organs may also be secondarily affected by any immune response against SARS-CoV-2 structural proteins.





Similar content being viewed by others
Data availability
All data availability statements were mentioned in methodology section.
References
Sen S, Kannan NB, Kumar J, et al. Retinal manifestations in patients with SARS-CoV-2 infection and pathogenetic implications: a systematic review. Int Ophthalmol. 2022;42(1):323–36. https://doi.org/10.1007/s10792-021-01996-7.
Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–440440.
Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;S0049–3848(20):30120–1.
Klok FA, Kruip MJHA, van der Meer NJM. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–50.
Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, et al. Cerebral venous thrombosis associated with COVID-19. Am J Neuroradiol. 2020;41:1370–6.
Artifoni M, Danic G, Gautier G, Gicquel P, Boutoille D, Raffi F, et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50(1):211–6.
Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50:54–67.
Marchetti M. COVID-19-driven endothelial damage: complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann Hematol. 2020;99:1701–7.
Casagrande M, Fitzek A, Pu¨schel K, et al. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm. 2020;28:721–5.
Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–8.
Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62.
Marinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort R Jr. Retinal findings in patients with COVID-19. Lancet. 2020;395(10237):1610. https://doi.org/10.1016/S0140-6736(20)31014-X.
Schnichels S, Rohrbach JM, Bayyoud T, Thaler S, Ziemssen F, Hurst J. Kann SARS-CoV-2 das Auge infizieren? – Ein Überblicküber den Rezeptorstatus in okularemGewebe [Can SARS-CoV-2 infect the eye?-An overview of the receptor status in ocular tissue]. Ophthalmologe. 2020;117(7):618–21. https://doi.org/10.1007/s00347-020-01160-z.
Naughton A, Ong AY, Gkika T, Downes S. Bilateral paracentral acute middle maculopathy in a SARS-CoV-2-positive patient. Postgrad Med J. 2021;98(2):105–6. https://doi.org/10.1136/postgradmedj-2021-140500.
Landecho MF, Yuste JR, Gándara E, et al. COVID-19 retinal microangiopathy as an in vivo biomarker of systemic vascular disease? J Intern Med. 2021;289(1):116–20. https://doi.org/10.1111/joim.13156.
Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Progress Retinal Eye Res. 2013;34:19–48. https://doi.org/10.1016/j.preteyeres.2013.02.001.
Reglero-Real N, Colom B, Bodkin JV, Nourshargh S. Endothelial cell junctional adhesion molecules: role and regulation of expression in inflammation. ArteriosclerThrombVasc Biol. 2016;36(10):2048–57. https://doi.org/10.1161/ATVBAHA.116.307610.
Dong R, Pan S, Peng Z, Zhang Y, Yang J. mTM-align: a server for fast protein structure database search and multiple protein structure alignment. Nucleic Acids Res. 2018;46(W1):W380–6. https://doi.org/10.1093/nar/gky430.
**rui Xu, Zhang Yang. How significant is a protein structure similarity with TM-score = 0.5? Bioinformatics. 2010;26(7):889–95.
Zhang Y, Skolnick J. TM-align: A protein structure alignment algorithm based on TM-score. Nucleic Acids Res. 2005;33:2302–9.
Kurcinski M, Jamroz M, Blaszczyk M, Kolinski A, Kmiecik S. CABS-dock web server for the flexible docking of peptides to proteinswithout prior knowledge of the binding site. Nucleic Acids Res. 2015;43(W1):W419–24. https://doi.org/10.1093/nar/gkv456.
Blaszczyk M, Ciemny MP, Kolinski A, Kurcinski M, Kmiecik S. Protein–peptide docking using CABS-dock and contact information. Briefings Bioinformatics. 2019;20(6):2299–305. https://doi.org/10.1093/bib/bby080.
Kulkarni R, Behboudi S, Sharif S. Insights into the role of Toll-like receptors in modulation of T cell responses. Cell Tissue Res. 2011;343(1):141–52. https://doi.org/10.1007/s00441-010-1017-1.
Rego N, Koes D. 3Dmol.js: molecular visualization withWebGL. Bioinformatics. 2014;31:1322–4.
Hanson RM, Prilusky J, Renjian Z, Nakane T, Sussman JL. JSmol and the next-generation web-based representation of 3D molecular structure as applied toproteopedia. Isr J Chem. 2013;53:207–16.
Verdecchia P, Cavallini C, Spanevello A, Angeli F. COVID-19: ACE2 centric infective disease? Hypertension. 2020;76(2):294–9.
Yamaguchi Y, Moriki T, Igari A, Matsubara Y, Ohnishi T, Hosokawa K, Murata M. Studies of a microchip flowchamber system to characterize whole blood thrombogenicity in healthy individuals. Thromb Res. 2013;132:263–70.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383(2):120–8.
Campbell CM, Kahwash R. Will complement inhibition be the new target in treating COVID-19 related systemic thrombosis? Circulation. 2020;141:1739–41. https://doi.org/10.1161/CIRCULATIONAHA.120.047419.
Delabranche X, Helms J, Meziani F. Immunohaemostasis: a new view on haemostasis during sepsis. Ann Intensive Care. 2017;7:117.
Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217:e20200652.
Becker RC. Toward understanding the 2019 Coronavirus and its impact on the heart. J Thromb Thrombolysis. 2020. https://doi.org/10.1007/s11239-020-02107-6.
Caligiuri G. CD31 as a therapeutic target in atherosclerosis. Circ Res. 2020;126:1178–89.
Lertkiatmongkol P, Liao D, Mei H, Hu Y, Newman PJ. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). CurrOpinHematol. 2016;23(3):253–9. https://doi.org/10.1097/MOH.0000000000000239.
Zaremba J, Losy J. sPECAM-1 in serum and CSF of acute ischaemic stroke patients. Acta Neurol Scand. 2002;106:292–8.
Losy J, Niezgoda A, Wender M. Increased serum levels of soluble PECAM-1 in multiple sclerosis patients with brain gadolinium-enhancing lesions. J Neuroimmunol. 1999;99:169–72.
Bauer W, Ulke J, Galtung N, et al. Role of cell adhesion molecules for prognosis of disease development of patients with and without COVID-19 in the emergency department. J Infect Dis. 2021;223(8):1497–9. https://doi.org/10.1093/infdis/jiab042.
Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J ThrombHaemost. 2020;18:844–7.
Hooper LC, Chin MS, Detrick B, Hooks JJ. Retinal degeneration in experimental coronavirus retinopathy (ECOR) is associated with increased TNF-alpha, soluble TNFR2 and altered TNF-alpha signaling. J Neuroimmunol. 2005;166(1–2):65–74. https://doi.org/10.1016/j.jneuroim.2005.05.018.
Hooks JJ, Percopo C, Wang Y, Detrick B. Retina and retinal pigment epithelial cell autoantibodies are produced during murine coronavirus retinopathy. J Immunol. 1993;151(6):3381–9.
Karki R, Sharma BR, Tuladhar S, et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184(1):149-168.e17. https://doi.org/10.1016/j.cell.2020.11.025.
Zhu J, Pang J, Ji P, Zhong Z, Li H, Li B, et al. Elevated interleukin-6 is associated with severity of COVID-19: a meta-analysis. J Med Virol. 2020;93(1):35–7. https://doi.org/10.1002/jmv.26085.
Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. 2020;92:2283–5.
McConnell MJ, Kawaguchi N, Kondo R, et al. Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy. J Hepatol. 2021;75(3):647–58. https://doi.org/10.1016/j.jhep.2021.04.050.
Kang S, Tanaka T, Inoue H, et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci USA. 2020;117(36):22351–6. https://doi.org/10.1073/pnas.2010229117.
Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123–30. https://doi.org/10.1080/22221751.2020.1770129.
Zhao Y, Qin L, Zhang P, et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight. 2020;5(13):e139834. https://doi.org/10.1172/jci.insight.139834
Gadient RA, Patterson PH. Leukemia inhibitory factor, Interleukin 6, and other cytokines using the GP130 transducing receptor: roles in inflammation and injury. Stem Cells. 1999;17(3):127–37. https://doi.org/10.1002/stem.170127.[PubMed][CrossRef][GoogleScholar].
McKinstry KK, Strutt TM, Buck A, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182(12):7353–63. https://doi.org/10.4049/jimmunol.0900657.
Fattori E, Cappelletti M, Costa P, et al. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180(4):1243–50. https://doi.org/10.1084/jem.180.4.1243.
Amarante-Mendes GP, et al. Pattern recognition receptors and the host cell death molecular machinery. Front Immunol. 2018;9:764.
Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol Rev. 2008;223:271–83. https://doi.org/10.1111/j.1600-065x.2008.00630.x.[PubMed][CrossRef][GoogleScholar].
Mohammad Hosseini A, Majidi J, Baradaran B, Yousefi M. Toll-like receptors in the pathogenesis of autoimmune diseases. Adv Pharm Bull. 2015;5(1):605–14. https://doi.org/10.15171/apb.2015.082.
Afrin LB, Weinstock LB, Molderings GJ. COVID-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis. 2020;100:327–32. https://doi.org/10.1016/j.ijid.2020.09.016.
Khanmohammadi S, Rezaei N. Role of toll-like receptors in the pathogenesis of COVID-19. J Med Virol. 2021;93(5):2735–9. https://doi.org/10.1002/jmv.26826.
Cavassani KA, Ishii M, Wen H, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205(11):2609–21. https://doi.org/10.1084/jem.20081370[PMCfreearticle][PubMed][CrossRef][GoogleScholar].
Mazaleuskaya L, Veltrop R, Ikpeze N, Martin-Garcia J, Navas-Martin S. Protective role of toll-like receptor 3-induced type I interferon in murine coronavirus infection of macrophages. Viruses. 2012;4(5):901–23. https://doi.org/10.3390/v4050901[PMCfreearticle][PubMed][CrossRef][GoogleScholar].
Totura AL, Whitmore A, Agnihothram S, et al. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. MBio. 2015;6:3. https://doi.org/10.1128/mBio.00638-15[PMCfreearticle][PubMed][CrossRef][GoogleScholar].
Mukherjee R, Bhattacharya A, Bojkova D, et al. Famotidine inhibits toll-like receptor 3-mediated inflammatory signaling in SARS-CoV-2 infection. J Biol Chem. 2021;297(2):100925. https://doi.org/10.1016/j.jbc.2021.100925.
Biswas I, Khan GA. Coagulation disorders in COVID-19: role of toll-like receptors. J Inflamm Res. 2020;13:823–8. https://doi.org/10.2147/JIR.S271768
Oliveira-Nascimento L, Massari P, Wetzler LM. The role of TLR2 in infection and immunity. Front Immunol. 2012;3:79.
Khan S, Shafiei MS, Longoria C, Schoggins J, Savani RC, Zaki H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. eLife. 2021;10:e68563. https://doi.org/10.1101/2021.03.16.435700
Zheng M, Karki R, Williams EP, et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat Immunol. 2021;22(7):829–38. https://doi.org/10.1038/s41590-021-00937-x.
Author information
Authors and Affiliations
Contributions
Conception and design of study: IKK, LK, MK
Acquisition of data: IKK, MK
Analysis and/orinterpretation of data: IKK, MK, LK
Drafting the manuscript: IKK, EOT, LK, MM
Revising the manuscript critically for important intellectual content: IKK, LK, MM, RR
Approval of the version of the manuscript to be published: IKK, EOT, LK, MM, RR, AK, MK
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key messages
1. This research study evaluated potential immune molecular mimicry between the epitopes on the SARS-CoV19 S protein and retinal vascular endothelial cell proteins.
2. Bioinformatics was used to identify sequence similarities for potential immune response against the SARS-CoV19.
3. Significant protein sequence similarities between SARS-CoV19 and S-protein were found.
4. Retinal vascular endothelial proteins may have a risk of being targets of autoimmune attacks after infection with SARS-CoV19.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kutlutürk, I., Tokuç, E.Ö., Karabaş, L. et al. How the immune response to the structural proteins of SARS-CoV-2 affects the retinal vascular endothelial cells: an immune thrombotic and/or endotheliopathy process with in silico modeling. Immunol Res 72, 50–71 (2024). https://doi.org/10.1007/s12026-023-09412-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-023-09412-1