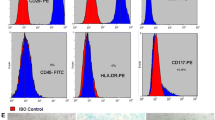
Abstract
Pluripotent, very small embryonic-like stem cells (VSELs) and tissue-committed ‘progenitors’ termed endometrial stem cells (EnSCs) are reported in mouse uterus. They express gonadal and gonadotropin hormone receptors and thus are vulnerable to early-life endocrine insults. Neonatal exposure of mouse pups to endocrine disruption cause stem/progenitor cells to undergo epigenetic changes, excessive self-renewal, and blocked differentiation that results in various uteropathies including non-receptive endometrium, hyperplasia, endometriosis, adenomyosis, and cancer-like changes in adult life. Present study investigated reversal of these uteropathies, by normalizing functions of VSELs and EnSCs. Two strategies were evaluated including (i) transplanting mesenchymal stromal cells (provide paracrine support) on D60 or (ii) oral administration of XAR (epigenetic regulator) daily from days 60–100 and effects were studied later in 100 days old mice. Results show normalization of stem/progenitor cells (Oct-4, Oct-4A, Sox-2, Nanog) and Wnt signalling (Wnt-4, β-catenin, Axin-2) specific transcripts. Flow cytometry results showed reduced numbers of 2–6 µm, LIN-CD45-SCA-1 + VSELs. Hyperplasia (Ki67) of epithelial (Pax-8, Foxa-2) and myometrial (α-Sma, Tgf-β) cells was reduced, adenogenesis (differentiation of glands) was restored, endometrial receptivity and differentiation (LIF, c-KIT, SOX-9, NUMB) and stromal cells niche (CD90, VIMENTIN, Pdgfra, Vimentin) were improved, cancer stem cells markers (OCT-4, CD166) were reduced while tumor suppressor genes (PTEN, P53) and epigenetic regulators (Ezh-2, Sirt-1) were increased. To conclude, normalizing VSELs/EnSCs to manage uteropathies provides a novel basis for initiating clinical studies. The study falls under the umbrella of United Nations Sustainable Development Goal 3 to ensure healthy lives and well-being for all of all ages.
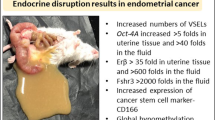
Graphical Abstract













Similar content being viewed by others
Data Availability
All relevant data is included in the manuscript and the supplement.
Code Availability
Not applicable.
Abbreviations
- VSELs :
-
Very Small embryonic-like stem cells
- EnSCs :
-
Endometrial stem progenitor cells
- MSC :
-
Mesenchymal stem/stromal cell
- DES :
-
Diethylstilbesterol
- E2 :
-
Estradiol
- EDCs :
-
Endocrine disrupting chemicals
- PND :
-
Postnatal day
- OCT-4 :
-
Octamer-binding transcription factor 4
- SOX-2 :
-
Sex determining region Y-box 2
- NANOG :
-
Homeobox transcription factor
- SCA-1 :
-
Stem cells antigen-1
- SSEA-1 :
-
Stage-specific embryonic antigen-1
- LIF :
-
Leukemia inhibitory factor
- c-KIT :
-
Receptor tyrosine kinase
- CSCs :
-
Cancer stem cells
- CD166 (ALCAM) :
-
Activated leukocyte cell adhesion molecule
- GFP :
-
Green fluorescent protein
- α-SMA :
-
Alpha-smooth muscle actin
- 7AAD :
-
7-Amino-actinomycin D
- PAX-8 :
-
Paired-box gene 8
- FOXA-2 :
-
Forkhead box protein A2
- NUMB :
-
NUMB: endocytic adaptor protein
- CD90 :
-
Cluster of Differentiation 90
- Ki-67 :
-
Marker of proliferation
- PTEN :
-
Phosphatase and TENsin homolog deleted on chromosome10
- P53 :
-
Tumour suppressor protein p53
- SIRT-1 :
-
Silent mating type information regulation 2 homolog-1
- DNMTs :
-
DNA methyltransferases
References
Skakkebæk, N. E., Lindahl-Jacobsen, R., Levine, H., Andersson, A. M., Jorgensen, N., Main, K. M., & Juul, A. (2022). Environmental factors in declining human fertility. Nature Reviews Endocrinology, 18, 139–157. https://doi.org/10.1038/s41574-021-00598-8
Murphy, A. R., Campo, H., & Kim, J. J. (2022). Strategies for modelling endometrial diseases. Nature Reviews Endocrinology, 18, 727–743. https://doi.org/10.1038/s41574-022-00725-z
Dutta, S., Banu, S. K., & Arosh, J. A. (2023). Endocrine disruptors and endometriosis. Reproductive Toxicology, 115, 56–73. https://doi.org/10.1016/j.reprotox.2022.11.007
Stephens, V. R., Rumph, J. T., Ameli, S., Bruner-Tran, K. L., & Osteen, K. G. (2022). The potential relationship between environmental endocrine disruptor exposure and the development of endometriosis and adenomyosis. Frontiers in Physiology, 12, 807685. https://doi.org/10.3389/fphys.2021.807685
Zhang, Y., Lu, Y., Ma, H., Xu, Q., & Wu, X. (2021). Combined exposure to multiple endocrine disruptors and uterine leiomyomata and endometriosis in US women. Frontiers in Endocrinology, 12, 726876. https://doi.org/10.3389/fendo.2021.726876
Bariani, M. V., Rangaswamy, R., Siblini, H., Yang, Q., Al-Hendy, A., & Zota, A. R. (2020). The role of endocrine-disrupting chemicals in uterine fibroid pathogenesis. Current Opinion in Endocrinology Diabetes & Obesity, 27, 380–387. https://doi.org/10.1097/MED.0000000000000578
Mallozzi, M., Leone, C., Manurita, F., Bellati, F., & Caserta, D. (2017). Endocrine disrupting chemicals and endometrial cancer: An overview of recent laboratory evidence and epidemiological studies. International Journal of Environmental Research and Public Health, 14, 334. https://doi.org/10.3390/ijerph14030334
Caserta, D., Costanzi, F., Marco, M., Benedetto, L., Matteucci, E., Assorgi, C., et al. (2021). Effects of endocrine-disrupting chemicals on endometrial receptivity and embryo implantation: A systematic review of 34 mouse model studies. International Journal of Environmental Research and Public Health, 18, 6840. https://doi.org/10.3390/ijerph18136840
Kyo, S., Sato, S., & Nakayama, K. (2020). Cancer-associated mutations in normal human endometrium: Surprise or expected? Cancer Science, 111, 3458–3467. https://doi.org/10.1111/cas.14571
Guo, S. W. (2020). Cancer-associated mutations in endometriosis: Shedding light on the pathogenesis and pathophysiology. Human Reproduction Update, 26, 423–449. https://doi.org/10.1093/humupd/dmz047
Suda, K., Nakaoka, H., Yoshihara, K., Ishiguro, T., Tamura, R., Mori, Y., Suda, K., Nakaoka, H., Yoshihara, K., Ishiguro, T., Tamura, R., Mori, Y., Yamawaki, K., Adachi, S., Takahashi, T., Kase, H., Tanaka, K., Yamamoto, T., Motoyama, T., … Enomoto, T. (2018). Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell Reports, 24, 1777–1789. https://doi.org/10.1016/j.celrep.2018.07.037
Anglesio, M. S., Papadopoulos, N., Ayhan, A., Nazeran, T. M., Noë, M., Horlings, et al. (2017). Cancer-associated mutations in endometriosis without cancer. New England Journal of Medicine, 376, 1835–1848. https://doi.org/10.1056/NEJMoa1614814
Singh, P., & Bhartiya, D. (2023). Mouse uterine stem cells are affected by endocrine disruption and initiate uteropathies. Reproduction, 165, 249–268. https://doi.org/10.1530/REP-22-0337
Cousins, F. L., Pandoy, R., **, S., & Gargett, C. E. (2021). The elusive endometrial epithelial stem/progenitor cells. Frontiers in Cell and Developmental Biology, 9, 640319. https://doi.org/10.3389/fcell.2021.640319
Santamaria, X., Mas, A., Cervelló, I., Taylor, H., & Simon, C. (2018). Uterine stem cells: From basic research to advanced cell therapies. Human Reproduction Update, 24, 673–693. https://doi.org/10.1093/humupd/dmy028
Gao, S., Zhang, Y., Liang, K., Bi, R., & Du, Y. (2022). Mesenchymal stem cells (MSCs): A novel therapy for type 2 diabetes. Stem Cells International, 2022, 1–17. https://doi.org/10.1155/2022/8637493
Rungsiwiwut, R., Virutamasen, P., & Pruksananonda, K. (2021). Mesenchymal stem cells for restoring endometrial function: An infertility perspective. Reproductive Medicine and Biology, 20, 13–19. https://doi.org/10.1002/rmb2.12339
Syed, S. M., Kumar, M., Ghosh, A., Tomasetig, F., Ali, A., Whan, R. M., Syed, S. M., Kumar, M., Ghosh, A., Tomasetig, F., Ali, A., Whan, R. M., Alterman, D., & Tanwar, P. S. (2020). Endometrial Axin2 + cells drive epithelial homeostasis, regeneration, and cancer following oncogenic transformation. Cell Stem Cell, 26, 64-80e13. https://doi.org/10.1016/j.stem.2019.11.012
James, K., Bhartiya, D., Ganguly, R., Kaushik, A., Gala, K., Singh, P., & Metkari, S. M. (2018). Gonadotropin and steroid hormones regulate pluripotent very small embryonic-like stem cells in adult mouse uterine endometrium. Journal of Ovarian Research, 11, 83. https://doi.org/10.1186/s13048-018-0454-4
Gunjal, P., Bhartiya, D., Metkari, S., Manjramkar, D., & Patel, H. (2015). Very small embryonic-like stem cells are the elusive mouse endometrial stem cells- a pilot study. Journal of Ovarian Research, 8, 9. https://doi.org/10.1186/s13048-015-0138-2
Ratajczak, M. Z., Ratajczak, J., & Kucia, M. (2019). Very small embryonic-like stem cells (vsels). Circulation Research, 124, 208–210. https://doi.org/10.1161/CIRCRESAHA.118.314287
Bhartiya, D., Shaikh, A., Anand, S., Patel, H., Kapoor, S., Sriraman, K., … Unni, S. (2016). Endogenous, very small embryonic-like stem cells: Critical review, therapeutic potential and a look ahead. Human Reproduction Update, 23, 1–36. https://doi.org/10.1093/humupd/dmw030
Singh, P., & Bhartiya, D. (2021). Pluripotent stem (VSELs) and progenitor (EnSCs) cells exist in adult mouse uterus and show cyclic changes across estrus cycle. Reproductive Sciences, 28, 278–290. https://doi.org/10.1007/s43032-020-00250-2
Singh, P., Metkari, S., & Bhartiya, D. (2022). Additional evidence to support OCT-4 positive VSELs and EnSCs as the elusive tissue-resident stem/progenitor cells in adult mice uterus. Stem Cell Research & Therapy, 13, 60. https://doi.org/10.1186/s13287-022-02703-8
Singh, P., Metkari, S. M., & Bhartiya, D. (2022). Mice uterine stem cells are affected by neonatal endocrine disruption & initiate uteropathies in adult life independent of circulatory ovarian hormones. Stem Cell Reviews and Reports, 18, 1686–1701. https://doi.org/10.1007/s12015-021-10279-8
Fernandes, G., Silva, G., Pavan, A., Chiba, D., Chin, C., & Santos, D. (2017). Epigenetic regulatory mechanisms induced by resveratrol. Nutrients, 9, 1201. https://doi.org/10.3390/nu9111201
Esfandyari, S., Chugh, R. M., Park, H., Hobeika, E., Ulin, M., & Al-Hendy, A. (2020). Mesenchymal stem cells as a bio-organ for treatment of female infertility. Cells, 9, 2253. https://doi.org/10.3390/cells9102253
Pokrovskaya, L. A., Zubareva, E. V., Nadezhdin, S. V., Lysenko, A. S., & Litovkina, T. L. (2020). Biological activity of mesenchymal stem cells secretome as a basis for cell-free therapeutic approach. Research Results in Pharmacology, 6, 57–68. https://doi.org/10.3897/rrpharmacology.6.49413
Abumaree, M. H., Jumah, A., Kalionis, M. A., Jawdat, B., Al Khaldi, D., AlTalabani, A., & Knawy, B. A. (2013). Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Reviews and Reports, 9, 16–31. https://doi.org/10.1007/s12015-012-9385-4
Gao, L., Huang, Z., Lin, H., Tian, Y., Li, P., & Lin, S. (2019). Bone marrow mesenchymal stem cells (bmscs) restore functional endometrium in the rat model for severe asherman syndrome. Reproductive Sciences, 26, 436–444. https://doi.org/10.1177/1933719118799201
Yi, K. W., Mamillapalli, R., Sahin, C., Song, J., Tal, R., & Taylor, H. S. (2019). Bone marrow-derived cells or C-X-C motif chemokine 12 (CXCL12) treatment improve thin endometrium in a mouse model†. Biology of Reproduction, 100, 61–70. https://doi.org/10.1093/biolre/ioy175
Santamaria, X., Cabanillas, S., Arbona, C., & Simon, C. (2015). Autologous cell therapy with CD133 + bone marrow stem cells in refractory Asherman’s syndrome and endometrial atrophy: A pilot cohort study. Fertility and Sterility, 104, e90. https://doi.org/10.1016/j.fertnstert.2015.07.278
Novakovic, R., Rajkovic, J., Gostimirovic, M., Gojkovic-Bukarica, L., & Radunovic, N. (2022). Resveratrol and reproductive health. Life, 12, 294. https://doi.org/10.3390/life12020294
Amaya, S. C., Savaris, R. F., Filipovic, C. J., Wise, J. D., Hestermann, E., Young, S. L., & Lessey, B. A. (2014). Resveratrol and endometrium: A closer look at an active ingredient of red wine using in vivo and in vitro models. Reproductive Sciences, 21, 1362–1369. https://doi.org/10.1177/1933719114525271
Wang, C., Chen, Z., Zhao, X., Lin, C., Hong, S., Lou, Y., **, Y., Wang, C., Chen, Z., Zhao, X., Lin, C., Hong, S., Lou, Y., Shi, X., Zhao, M., Yang, X., Guan, M.-X., & **, Y. (2021). Transcriptome-based analysis reveals therapeutic effects of resveratrol on endometriosis in a rat model. Drug Design Development and Therapy, Volume 15, 4141–4155. https://doi.org/10.2147/DDDT.S323790
Kong, X., Xu, X., Zhou, L., Zhu, M., Yao, S., Ding, Y., … Zhou, H. (2020). MTA1, a target of resveratrol, promotes epithelial-mesenchymal transition of endometriosis via ZEB2. Molecular Therapy - Methods & Clinical Development, 19, 295–306. https://doi.org/10.1016/j.omtm.2020.09.013
Kolahdouz Mohammadi, R., & Arablou, T. (2017). Resveratrol and endometriosis: In vitro and animal studies and underlying mechanisms (review). Biomedicine & Pharmacotherapy, 91, 220–228. https://doi.org/10.1016/j.biopha.2017.04.078
Chen, Lin, Shih, Wang, Hong, Shieh, … Hsia. (2019). Natural antioxidant resveratrol suppresses uterine fibroid cell growth and extracellular matrix formation in vitro and in vivo. Antioxidants, 8, 99. https://doi.org/10.3390/antiox8040099
Wu, C.-H., Shieh, T.-M., Wei, L.-H., Cheng, T.-F., Chen, H.-Y., Huang, T.-C., … Hsia,S.-M. (2016). Resveratrol inhibits proliferation of myometrial and leiomyoma cells and decreases extracellular matrix-associated protein expression. Journal of Functional Foods, 23, 241–252. https://doi.org/10.1016/j.jff.2016.02.038
Tripathi, V., Chhabria, S., Jadhav, V., Bhartiya, D., & Tripathi, A. (2018). Stem cells and progenitors in human peripheral blood get activated by extremely active resveratrol (XAR™). Stem Cell Reviews and Reports, 14, 213–222. https://doi.org/10.1007/s12015-017-9784-7
Singh, P., & Bhartiya, D. (2022). Molecular insights into endometrial cancer in mice. Stem Cell Reviews and Reports, 18, 1702–1717. https://doi.org/10.1007/s12015-022-10367-3
Chapman, J. C., Min, S. H., Freeh, S. M., & Michael, S. D. (2009). The estrogen-injected female mouse: New insight into the etiology of PCOS. Reproductive Biology and Endocrinology, 7, 47. https://doi.org/10.1186/1477-7827-7-47
Newbold, R. R., Bullock, B. C., & McLachlan, J. A. (1990). Uterine adenocarcinoma in mice following developmental treatment with estrogens: A model for hormonal carcinogenesis. Cancer Research, 50, 7677–7681.
Kaushik, A., Anand, S., & Bhartiya, D. (2020). Altered biology of testicular VSELs and SSCs by neonatal endocrine disruption results in defective spermatogenesis, reduced fertility and tumor initiation in adult mice. Stem Cell Reviews and Reports, 16, 893–908. https://doi.org/10.1007/s12015-020-09996-3
Sharma, D., & Bhartiya, D. (2022). Dysfunctional ovarian stem cells due to neonatal endocrine disruption result in pcos and ovarian insufficiency in adult mice. Stem Cell Reviews and Reports, 18, 2912–2927. https://doi.org/10.1007/s12015-022-10414-z
Soleimani, M., & Nadri, S. (2009). A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nature Protocols, 4, 102–106. https://doi.org/10.1038/nprot.2008.221
Lan, T., Luo, M., & Wei, X. (2021). Mesenchymal stem/stromal cells in cancer therapy. Journal of Hematology & Oncology, 14, 195. https://doi.org/10.1186/s13045-021-01208-w
Moraes, D. (2018). What the relationship between CD90 and CD44 in mesenchymal stem cells? Cytotherapy, 20, S47. https://doi.org/10.1016/j.jcyt.2018.02.124
Bhartiya, D., Singh, P., Sharma, D., & Kaushik, A. (2022). Very small embryonic-like stem cells (VSELs) regenerate whereas mesenchymal stromal cells (MSCs) rejuvenate diseased reproductive tissues. Stem Cell Reviews and Reports, 18, 1718–1727. https://doi.org/10.1007/s12015-021-10243-6
Kaushik, A., Metkari, S., Ali, S., & Bhartiya, D. (2023). Preventing/reversing adverse effects of endocrine disruption on mouse testes by normalizing tissue resident VSELs. Stem Cell Reviews and Reports. https://doi.org/10.1007/s12015-023-10601-6
Choi, H. Y., Seok, J., Kang, G. H., Lim, K. M., & Cho, S. G. (2021). The role of NUMB/NUMB isoforms in cancer stem cells. BMB Reports, 54, 335–343. https://doi.org/10.5483/BMBRep.2021.54.7.048
Brandmaier, A., Hou, S. Q., & Shen, W. H. (2017). Cell cycle control by PTEN. Journal of Molecular Biology, 429, 2265–2277. https://doi.org/10.1016/j.jmb.2017.06.004
Kim, D. K., Ham, M. H., Lee, S. Y., Shin, M. J., Kim, Y. E., Song, P., … Kim, J. H.(2020). CD166 promotes the cancer stem-like properties of primary epithelial ovarian cancer cells. BMB Reports, 53, 622–627. https://doi.org/10.5483/BMBRep.2020.53.12.102
Vannuccini, S., & Petraglia, F. (2019). Recent advances in understanding and managing adenomyosis. F1000Research, 8, 283. https://doi.org/10.12688/f1000research.17242.1
Johnatty, S. E., Stewart, C. J. R., Smith, D., Nguyen, A., O’ Dwyer, J., O’Mara, T.A., … Spurdle, A. B. (2020). Co-existence of leiomyomas, adenomyosis and endometriosis in women with endometrial cancer. Scientific Reports, 10, 3621. https://doi.org/10.1038/s41598-020-59916-1
Uimari, O., Nazri, H., & Tapmeier, T. (2021). Endometriosis and uterine fibroids (leiomyomata): Comorbidity, risks and implications. Frontiers in Reproductive Health, 3, 750018. https://doi.org/10.3389/frph.2021.750018
McMellen, A., Woodruff, E. R., Corr, B. R., Bitler, B. G., & Moroney, M. R. (2020). Wnt signaling in gynecologic malignancies. International Journal of Molecular Sciences, 21, 4272. https://doi.org/10.3390/ijms21124272
Fatima, I., Barman, S., Rai, R., Thiel, K. W., & Chandra, V. (2021). Targeting wnt signaling in endometrial cancer. Cancers, 13, 2351. https://doi.org/10.3390/cancers13102351
Nees, L. K., Heublein, S., Steinmacher, S., Juhasz-Böss, I., Brucker, S., Tempfer, C. B., & Wallwiener, M. (2022). Endometrial hyperplasia as a risk factor of endometrial cancer. Archives of Gynecology and Obstetrics, 306, 407–421. https://doi.org/10.1007/s00404-021-06380-5
Ring, K. L., Mills, A. M., & Modesitt, S. C. (2022). Endometrial hyperplasia. Obstetrics and gynecology, 140, 1061–1075. https://doi.org/10.1097/AOG.0000000000004989
Kim, H., Kim, H. J., & Ahn, H. S. (2023). Does endometriosis increase the risks of endometrial hyperplasia and endometrial cancer? Gynecologic Oncology, 169, 147–153. https://doi.org/10.1016/j.ygyno.2022.06.021
Kuai, D., Tang, Q., Tian, W., & Zhang, H. (2023). Rapid identification of endometrial hyperplasia and endometrial endometrioid cancer in young women. Discover Oncology, 14, 121. https://doi.org/10.1007/s12672-023-00736-w
Devis, L., Moiola, C. P., Masia, N., Martinez-Garcia, E., Santacana, M., Stirbat, T. V., … Colas, E. (2017). Activated leukocyte cell adhesion molecule (ALCAM) is a marker of recurrence and promotes cell migration, invasion, and metastasis in early-stage endometrioid endometrial cancer. The Journal of Pathology, 24, 475–487. https://doi.org/10.1002/path.4851
Liang, S., Huang, C., Jia, S., & Wang, B. (2011). Activated leukocyte cell adhesion molecule expression is up-regulated in the development of endometrioid carcinoma. International Journal of Gynecologic Cancer, 21, 523–528. https://doi.org/10.1097/IGC.0b013e31820e135a
**ao, M., Wang, X., Yan, M., & Chen, W. (2016). A systematic evaluation for the potential translation of CD166-related expression as a cancer biomarker. Expert Review of Molecular Diagnostics, 16, 925–932. https://doi.org/10.1080/14737159.2016.1211932
Bhartiya, D., Sharma, N., Dutta, S., Kumar, P., Tripathi, A., & Tripathi, A. (2023). Very small embryonic-like stem cells transform into cancer stem cells and are novel candidates for detecting/monitoring cancer by a simple blood test. Stem Cells, 41, 310–318. https://doi.org/10.1093/stmcls/sxad015
Capezzuoli, T., Rossi, M., La Torre, F., Vannuccini, S., & Petraglia, F. (2022). Hormonal drugs for the treatment of endometriosis. Current Opinion in Pharmacology, 67, 102311. https://doi.org/10.1016/j.coph.2022.102311
Kailasam, A., & Langstraat, C. (2022). Contemporary use of hormonal therapy in endometrial cancer: A literature review. Current Treatment Options in Oncology, 23, 1818–1828. https://doi.org/10.1007/s11864-022-01031-6
Kaushik, A., & Bhartiya, D. (2020). Additional evidence to establish existence of two stem cell populations including VSELs and SSCs in adult mouse testes. Stem Cell Reviews and Reports, 16, 992–1004. https://doi.org/10.1007/s12015-020-09993-6
Cui, X., Zhao, X., & Liang, Y. (2022). Sex differences in normal and malignant hematopoiesis. Blood Science, 4(4), 185–191. https://doi.org/10.1097/bs9.0000000000000133
Ratajczak, M. Z. (2017). Why are hematopoietic stem cells so ‘sexy’? On a search for developmental explanation. Leukemia, 31, 1671–1677. https://doi.org/10.1038/leu.2017.148
Mierzejewska, K., Borkowska, S., Suszynska, E., Suszynska, M., Poniewierska-Baran,A., Maj, M., … Ratajczak, M. Z. (2015). Hematopoietic stem/progenitor cells express several functional sex hormone receptors—novel evidence for a potential developmental link between hematopoiesis and primordial germ cells. Stem Cells and Development, 24, 927–937. https://doi.org/10.1089/scd.2014.0546
Squillaro, T., Peluso, G., & Galderisi, U. (2016). Clinical trials with mesenchymal stem cells: An update. Cell Transplantation, 25, 829–848. https://doi.org/10.3727/096368915X689622
Wang, Y.-J., Zhao, P., Sui, B.-D., Liu, N., Hu, C.-H., Chen, J., … **, Y. (2018).Resveratrol enhances the functionality and improves the regeneration of mesenchymal stem cell aggregates. Experimental & Molecular Medicine, 50, 1–15. https://doi.org/10.1038/s12276-018-0109-y
Suvorova, I. I., Knyazeva, A. R., Petukhov, A. V., Aksenov, N. D., & Pospelov, V. A. (2019). Resveratrol enhances pluripotency of mouse embryonic stem cells by activating AMPK/Ulk1 pathway. Cell Death Discovery, 5, 61. https://doi.org/10.1038/s41420-019-0137-y
Bhartiya, D. (2013). Are mesenchymal cells indeed pluripotent stem cells or just stromal cells? OCT-4 and VSELs biology has led to better understanding. Stem Cells International, 2013, 1–6. https://doi.org/10.1155/2013/547501
Taichman, R. S., Wang, Z., Shiozawa, Y., Jung, Y., Song, J., Balduino, A., … Krebsbach,P. H. (2010). Prospective identification and skeletal localization of cells capable of multilineage differentiation in vivo. Stem Cells and Development, 19, 1557–1570. https://doi.org/10.1089/scd.2009.0445
Bhartiya, D., Mohammad, S. A., Singh, P., Sharma, D., & Kaushik, A. (2022). GFP tagged VSELs help delineate novel stem cells biology in multiple adult tissues. Stem Cell Reviews and Reports, 18, 1603–1613. https://doi.org/10.1007/s12015-022-10401-4
Ren, G., Shi, J., Huang, S., Liu, C., Ni, F., He, Y., … **e, H. (2022). The fabrication of novel zein and resveratrol covalent conjugates: Enhanced thermal stability, emulsifying and antioxidant properties. Food Chemistry, 374, 131612. https://doi.org/10.1016/j.foodchem.2021.131612
Berretta, M., Bignucolo, A., Di Francia, R., Comello, F., Facchini, G., Ceccarelli,M., … Maurea, N. (2020). Resveratrol in cancer patients: from bench to bedside. International Journal of Molecular Sciences, 21, 2945. https://doi.org/10.3390/ijms21082945
Bhaskara, V. K., Mittal, B., Mysorekar, V. V., Amaresh, N., & Simal-Gandara, J. (2020). Resveratrol, cancer and cancer stem cells: A review on past to future. Current Research in Food Science, 3, 284–295. https://doi.org/10.1016/j.crfs.2020.10.004
Madanes, D., Meresman, G., Valla, S. A., Hassan, N., Kiesel, L., Greve, B., … Ricci,A. G. (2022). Resveratrol impairs cellular mechanisms associated with the pathogenesis of endometriosis. Reproductive BioMedicine Online, 44, 976–990. https://doi.org/10.1016/j.rbmo.2022.02.008
Ren, B., Kwah, M. X.-Y., Liu, C., Ma, Z., Shanmugam, M. K., Ding, L., … Goh, B. C. (2021) . Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Letters, 515, 63–72. https://doi.org/10.1016/j.canlet.2021.05.001
Ko, J.-H., Sethi, G., Um, J.-Y., Shanmugam, M. K., Arfuso, F., Kumar, A. P., … Ahn,K. S. (2017). The role of resveratrol in cancer therapy. International Journal of Molecular Sciences, 18, 2589. https://doi.org/10.3390/ijms18122589
Hmadcha, A., Martin-Montalvo, A., Gauthier, B. R., Soria, B., & Capilla-Gonzalez, V. (2020). Therapeutic potential of mesenchymal stem cells for cancer therapy. Frontiers in Bioengineering and Biotechnology, 8, 43. https://doi.org/10.3389/fbioe.2020.00043
Li, J., Qi, J., Yao, G., Zhu, Q., Li, X., Xu, R., … Sun, Y. (2021). Deficiency of Sirtuin 1 impedes endometrial decidualization in recurrent implantation failure patients. Frontiers in Cell and Developmental Biology, 9:598364. https://doi.org/10.3389/fcell.2021.598364
Cummings, M. J., Yu, H., Paudel, S., Hu, G., Li, X., Hemberger, M., & Wang, X. (2022). Uterine-specific SIRT1 deficiency confers premature uterine aging and impairs invasion and spacing of blastocyst, and stromal cell decidualization, in mice. Molecular Human Reproduction, 28, gaac016. https://doi.org/10.1093/molehr/gaac016
Taguchi, A., Wada-Hiraike, O., Kawana, K., Koga, K., Yamashita, A., Shirane, A., & Fujii, T. (2014). Resveratrol suppresses inflammatory responses in endometrial stromal cells derived from endometriosis: A possible role of the sirtuin 1 pathway. Journal of Obstetrics and Gynaecology Research, 40, 770–778. https://doi.org/10.1111/jog.12252
Shirane, A., Wada-Hiraike, O., Tanikawa, M., Seiki, T., Hiraike, H., Miyamoto, Y., Taketani, Y., Shirane, A., Wada-Hiraike, O., Tanikawa, M., Seiki, T., Hiraike, H., Miyamoto, Y., Sone, K., Hirano, M., Oishi, H., Oda, K., Kawana, K., Nakagawa, S., … Taketani, Y. (2012). Regulation of SIRT1 determines initial step of endometrial receptivity by controlling E-cadherin expression. Biochemical and Biophysical Research Communications, 424, 604–610. https://doi.org/10.1016/j.bbrc.2012.06.160
Elibol, B., & Kilic, U. (2018). High levels of SIRT1 expression as a protective mechanism against disease-related conditions. Frontiers in Endocrinology, 9, 614. https://doi.org/10.3389/fendo.2018.00614
Tatone, C., Di Emidio, G., Barbonetti, A., Carta, G., Luciano, A. M., Falone, S., & Amicarelli, F. (2018). Sirtuins in gamete biology and reproductive physiology: Emerging roles and therapeutic potential in female and male infertility. Human Reproduction Update, 24, 267–289. https://doi.org/10.1093/humupd/dmy003
Seishima, R., Leung, C., Yada, S., Murad, K. B. A., Tan, L. T., Hajamohideen, A.,… Barker, N. (2019). Neonatal Wnt-dependent Lgr5 positive stem cells are essential for uterine gland development. Nature Communications, 10, 5378. https://doi.org/10.1038/s41467-019-13363-3
Chumduri, C., & Turco, M. Y. (2021). Organoids of the female reproductive tract. Journal of Molecular Medicine, 99, 531–553. https://doi.org/10.1007/s00109-020-02028-0
Lõhmussaar, K., Boretto, M., & Clevers, H. (2020). Human-derived model systems in gynecological cancer research. Trends in Cancer, 6, 1031–1043. https://doi.org/10.1016/j.trecan.2020.07.007
Acknowledgements
Help from Confocal Microscopy; Flow Cytometry and Histology Central Facilities at NIRRCH is acknowledged. Subhan MD help is acknowledged for XAR treatment. We acknowledge all those who have published data that may be directly relevant but may not have been quoted.
Funding
The study was supported by the core support provided by Indian Council of Medical Research, Government of India, New Delhi. PS acknowledges the DST-INSPIRE fellowship (IF170144).
Author information
Authors and Affiliations
Contributions
DB planned the study, arrange the funds, and helped in manuscript drafting. All authors discussed the findings, read, and approved the final version. PS helped design the study, performed all experiments, and wrote the article. SMM performed all the surgeries. AT provided XAR- a nano-formulation of Resveratrol.
Corresponding author
Ethics declarations
Ethics Approval
Project no-16/17 was approved on 21 December 2017.
Consent to Participate
Not applicable.
Consent for Publication
NIRRCH manuscript number RA/1420/12-2022.
Conflict of Interest
Authors declare no conflict of interest whatsoever that could be perceived as prejudicing the impartiality of the research reported. This study was completed when DB was at NIRRCH and no conflict of interest existed with Epigeneres Biotech Pvt Ltd., Mumbai which she joined after superannuation. XAR was provided as a gift by Epigeneres as an outcome of an earlier collaborative publication (https://doi.org/10.1007/s12015-017-9784-7).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All work is done at NIRRCH.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 3.29 MB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, P., Metkari, S.M., Tripathi, A. et al. Reversing Uteropathies Including Cancer-Like Changes in Mice by Transplanting Mesenchymal Stromal Cells or XAR Treatment. Stem Cell Rev and Rep 20, 258–282 (2024). https://doi.org/10.1007/s12015-023-10632-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10632-z