Abstract
Tooth defect and tooth loss are common clinical diseases in stomatology. Compared with the traditional oral restoration treatment, tooth regeneration has unique advantages and is currently the focus of oral biomedical research. It is known that dozens of cytokines/growth factors and other bioactive factors are expressed in a spatial-temporal pattern during tooth development. On the other hand, the technology for spatial-temporal control of drug release has been intensively studied and well developed recently, making control release of these bioactive factors mimicking spatial-temporal pattern more feasible than ever for the purpose of tooth regeneration. This article reviews the research progress on the tooth development and discusses the future of tooth regeneration in the context of spatial-temporal release of developmental factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Several cytokines/growth factors are involved in the precise and directional development of specific tissues and organs. In the craniomaxillofacial region, the development of teeth depends largely on the orderly interaction between the ectodermal epithelium and the mesenchyme [1].
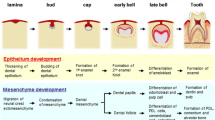
The tooth development process is generally divided into the initiation stage, the bud stage, the cap stage and the bell stage (Fig. 1). At the initiation stage, the epithelial tissue known as the dental placode, locally thickens, and continues to develop into the tooth bud [2]. Meanwhile, the mesenchymal tissue near the tooth bud, aggregates to form the tooth germ. Through the proliferation and folding of the epithelial tissue, the buds gradually evolve to the cap and bell stages. Clusters of undifferentiated epithelial cells, known as the enamel knot, can be observed at the center of the inner enamel epithelium. Each tooth germ has only one primary enamel knot. When the primary enamel knot disappears, secondary enamel knots will appear at the prospective apex of the molars. The enamel knot is considered to be the signal center that controls the shape of the cusp [3]. Subsequently, the epithelial tissue forms odontoblasts and ameloblasts, that lead to the formation of the dentin and the enamel, respectively. After the crown formation, the cervical loop of the dental epithelial cells, continues to elongate and forms a double-layered epithelial structure, found between the dental follicle and the dental papilla, and named the Hertwig’s epithelial root sheath (HERS). Conventionally, researchers believe that HERS is the signal center of the root formation [4].
Many studies have shown that cytokines/growth factors such as BMPs, FGFs, SHHs, WNTs and TNFs, play an important role during this process [1]. Moreover, the expression of these cytokines is characterized by a spatial-temporal specificity [5,6,7] (Fig. 1). Aberrant expression may lead to tooth development abnormalities [1]. The spatio-temporal control of the developmental cues might be the future for tooth regeneration applications.
With advances in developmental biology and drug delivery, tooth regeneration would be more promising than ever before (Fig. 2). In the following sections, we summarize recent advances in developmental biology and discuss the clues for tooth regeneration in the context of the spatial-temporal control of bioactive drug release.
Schematic representation of the bio-inspired dental regeneration strategy. The gene expression pattern during tooth development is obtained by biology and bioinformatics, and the development associated with spatial-temporal specific expression could be approached by using different control release strategies for regeneration purpose, and making the goal of tooth regeneration expectable
Cytokines/ Growth Factors and Tooth Development
BMP, FGF, WNT and SHH signaling pathways are known signaling pathways in tooth development (Tables 1 and 2). Recently, other signaling pathways, such as TNF [8], YAP-Hippo [9] and mTORC1 [35]. It is also expressed in the surrounding inner enamel epithelium and in the stratum intermedium cells during the following stages [36]. The decrease or loss of SHH expression leads to a cap stage tooth rudiment, which has a severely disrupted morphology [37]. SHH also plays vital roles in the development of periodontal tissue [38]. As described above, BMP, WNT and SHH signals are interconnected during tooth development. The differential fate of epithelial stem cells, in mouse molars and incisors, is defined by BMP/SHH signaling network [39]. When reducing SHH function in the epithelium, WNT and FGF signaling are upregulated [40].
Other Factors
The EDA (ectodysplasin A)-EDAR (ectodysplasin A receptor) system has also been found to be involved in tooth development. It regulates interactions within or between epithelial and mesenchymal cells, and tissues functions by controlling NF-κB-mediated transcription of effectors or inhibitors of the WNT, SHH, FGF and TGF-β pathways [41]. Mutation in Tabby and identified as Ectodysplasin A1 (EDAA1), displays a characterized tooth phenotype, associated with significant reduction in the size and number of molar cusps, and frequent absence of incisors and third molar in the studied mice [42]. Another recent study suggested that EDA mutations cause non-syndromic tooth agenesis [43].
Dental Regeneration Via Reactivating the Developmental Cues
Dental regeneration medicine represents an attractive multidisciplinary approach that offsets traditional dental restoration techniques. As mentioned above, a variety of cytokines participate in different stages of tooth development and in a spatial-temporal manner [1]. The control release of the cytokines for dental regeneration is appealing and is being implemented. Its development depends on research progress in biomaterials, stem cell biology and in other scientific technologies (Fig. 3).
Strategies for tooth regeneration by reactivating developmental cues. A Different control release strategies of secretory factors based on biological materials. a) Self-degradation; b) pH-responsive release; c) Magnetic release; d) Thermal release; e) 3D printing. B Small RNAs are involved in different parts of the gene expression process. C Different turn-on/off systems for spatial-temporal control of gene expression. D In vivo delivery of gene expression system. E Transplantation of genetically modified cells. FUnder the above strategies, cells from different sources can be directed to differentiate into specific cells and eventually achieve tooth regeneration
Control Release of Secretory Factors
Biomaterial Based Control of Secretory Factors Releases
Self-degradation is based on the rate of materials degradation in a specific physiological environment, to achieve the spatial-temporal sustained release of cytokines (Fig. 3A a). Although this technique has been widely used in tissue engineering scaffolds, traditional techniques have significant drawbacks, such as high initial release and low bioactive molecular activity. In order to inhibited the burst release of cytokines and enhanced structural stability, many scholars are committed to inventing various kinds of better materials. Fahmy and his co-workers used a low dose of rBMP2 loaded on a resorbable bioactive ceramic to accelerated bone regeneration [44]. Recently, chirality-controlled enzyme-responsive protein nanocapsules were shown to alter the degradation rate by changing the constituent ratio of the material composition, resulting in enhancing wound healing and tissue repair in vivo via the delivery of multiple proteins in a spatiotemporal manner [45]. Affinity interaction is an alternative strategy to achieve sustained release of cytokines. In tissue engineering, the most common way to improve the release kinetics is through heparin-immobilized scaffolds that immobilize cytokines [46]. Wu et al. showed that heparin-based coacervate of FGF2 played a synergistic role with cell proliferation and endogenous facilitated VEGF in improving skin wound healing [3A e) [59]. The flexibility and controllability of 3D bioprinting enable complex and customized release profiles of multiple cytokines to achieve spatial-temporal gradients that regulate cellular functions in tissue or organ regeneration [60, 61]. Moreover, many studies have promoted the application of 3D printing technology in cytokine sustained-release by improving processing [62], advancing technology [63] or allowing combinations with other forms of carriers [64]. Up to now, these materials have been successfully used in various tissue and organ regeneration experiments in vitro and in vivo, such as vascular regeneration [65], bone regeneration [63] and skin regeneration [66]. The 4D printing technology is a dynamic and time dependent manufacturing process based on advanced 3D-print features, which providing great potential for tissue and organ engineering applications [67].
Control Delivery of Small RNAs
Small RNAs including small interfering RNAs (siRNAs) and microRNAs (miRNAs), are part of the short chain RNAs in non-coding RNAs (ncRNAs) (Fig. 3B). SiRNAs are double-stranded RNAs that downregulate gene expression guided by sequence complementarity with the target mRNA. Since its first discovery in 1998 [68], its delivery strategy has developed rapidly. So far, many different siRNA delivery approaches including siRNA conjugates and lipid nanoparticles, have been applied to disease treatment and tissue regeneration [69]. For example, Zhang et al. developed a targeting system for delivering siRNAs to markedly promoted bone formation [70]. More recently, Castleberry et al. developed an ultrathin polymer coating to sustain the local delivery of siRNA so as to improve wound healing in diabetic mice [71]. Furthermore, the potential toxicities of these technology have been gradually discovered. These include but not limited to on-target effects, sequence-specific off-target effects, immune activation and toxicity associated with the delivery vehicles [72].
MiRNAs can simultaneously identify hundreds of target mRNAs with multiple miRNAs working together for the same mRNA [73]. A As post-transcriptional gene regulators, they can target and disassemble mRNAs or repress their translation [74]. Many studies have shown that miRNAs play a significant regulatory role in tissue repair and regeneration, such as wound healing [75], cardiac repair [76]. In vivo delivery of exogenous miRNAs provides an effective way to regulate gene expression during tissue repair and regeneration, which was proved and validated in mice [77] and zebrafishs [78]. To optimize miRNA delivery, Zhang et al. developed a cell-free 3D scaffold with biodegradable microspheres, that spatially regulated the release of miR-26a to repair critically-sized bone defects in osteoporotic mice [79]. Zhou et al. used miR-126-loaded electrospun membranes for miRNAs local delivery to improve blood vessel regeneration [80]. Moreover, a recent study showed that intracardiac injection of a single administration of synthetic miRNA-lipid formulations enhanced cardiac repair in mice after myocardial infarction [81].
Spatial-Temporal Delivery of Gene Expression Systems
Delivery of gene expression systems that produce locally nascent proteins in vivo, is more advantageous compared to traditional methods for products delivery. In recent years, research on genes-controlled expression has rapidly developed. Some important and potential technologies will briefly be introduced below, and their combinations will also be discussed (Figure C-D).
Spatial-Temporal Control of Gene Expression
Hormone Induction
All kinds of hormones participate in development and regeneration stages. Steroid hormones function by binding to receptor proteins in the cytoplasm of target cells to form hormone-receptor complexes, which enter the nucleus and bind to specific chromosomal sites to regulate the transcription of specific genes. For example, estrogens play pivotal roles in various physiological processes, most of which are mediated by the estrogen receptors alpha (ERα), beta (ERβ) and G protein-coupled receptor 30 (GPR30). Many studies have used estrogen-inducible promoters to modify gene expression systems to regular gene expression [82,83,84]. Senturk et al. optimized a CRISPR/Cas9 system by combining it with an FKBP12-derived destabilizing domain and an inducible Cre-estrogen receptor fusion domain, which enabled rapid and tunable gene editing [85].
Optogenetics Regulation
Optogenetics is a rapidly develo** bioengineering technology which integrates many subjects, such as optics, software control technology, genetic engineering technology, electrophysiological technology. It was originally applied in the field of neurology and a recent review indicated that it could control nerve growth and neurotrophic factor expression in a precise spatial and temporal manner [86]. The light-based mechanisms can activate or inhibit the expression of target genes in the FGF [87], WNT/β-catenin [88] and TGF-β signaling pathways [89] by light-induced conformational change of various photoactivatable proteins or photocaging/uncaging of effectors [90]. Yang et al. created the LightON system, a light-switchable transgene system, which can initiate spatiotemporal expressions of target transgenes in mammalian cells, upon light stimulation [91]. However, potential toxicity associated with the high expression was reported by a study of zebrafish embryogenesis, which may limited its application [92]. To overcome this obstacle, the blue-light activated EL222 system, renamed TAEL was invented, and which drived the expression with minimal toxicity [93, 94]. In addition, some studies have used optical gene elements to link Cre recombinase to regulate DNA recombination [95, 96]. Recently, Nguyen et al. combined genetically encoded photo-switchable calcium actuators with dCas9 to control gene expression, overcoming some limitations of the CRISPR/Cas9 (dCas9) system [97]. Simultaneously, a CRISPR-dCas9 effector device that is activated by far-red light (FRL), engineered by Shao and his research team, efficiently promoted the differentiation of induced pluripotent stem cells (iPSCs) into functional neurons by up-regulating NEUROG2, a single neural transcription factor [98].
Dental Development-Related Specific Promoters
In the process of tooth development, some site-specific promoters like WNT1 promoter, play a vital role in regulating the orderly expression of genes. WNT1 encodes the signaling protein WNT1, involved in the canonical WNT pathway. Previous research has shown that the expression of WNT1 is restricted to the migrating neural crest cells, which contribute to tooth and mandible development [99]. Simultaneously, Chai et al. successfully constructed a transgenic model under the control of the WNT1 promoter [99]. Up to now, this conditional knockout model of transgenic mice has been widely used in the study of tooth development and regeneration [100,101,102].
In addition, dentin matrix protein 1 (DMP1) produced by odontoblasts and osteoblasts is mainly expressed in bone and dentin [103]. Jacob et al. showed that TCF11, which could specifically bind to the DMP1 promoter, played a significant role in regulating the transcription of DMP1 in odontoblasts and osteoblasts [103]. This provides a way to spatiotemporally regulate the expression of DMP1.
In Vivo Delivery of Gene Expression System
The in vivo gene delivery strategy can be generally divided into viral and non-viral vector delivery systems (Fig. 4). Viral vectors including oncoretroviruses, lentiviruses (LVs), adenoviruses (AVs) and adeno-associated viruses (AAVs), have relatively high efficiency. Initially, they are widely used in changing the expression of specific genes in vivo and in vitro [104]. In contrast to LVs, the nonintegrated DNA delivered by AAVs would be diluted during mitosis because of lack of integration machinery. However, it could be stably maintained in a nonintegrated form to mediate persistent gene expression in predominantly postmitotic cells [104]. With regard to damage repair and tissue regeneration, Eggers et al. used a lentiviral vector to regulate controlled expression of glial cell-line derived neurotrophic factor (GDNF), which exerts multiple effects on both Schwann cells and axons in the injured peripheral nerve [105]. Moreover, adenovirus-mediated WNT10b overexpression promoted hair follicle regeneration via the activation of the canonical WNT signaling pathway [ Yan, D. Z., Zhi, C., Yi, Q. S., Chao, L., & Yi, P. C. (2005). Making a tooth: Growth factors, transcription factors, and stem cells. Cell Research, 15(5), 301–316. Thiery, J. P., Duband, J. L., & Delouvee, A. (1982). Pathways and mechanisms of avian trunk neural crest cell migration and localization. Developmental Biology, 93(2), 324–343. Thesleff, I., & Mikkola, M. (2002). The role of growth factors in tooth development. International Review of Cytology, 217, 93–135. Ten Cate, A. R. (1996). The role of epithelium in the development, structure and function of the tissues of tooth support. Oral Diseases, 2(1), 55–62. Li, Z., Yu, M., & Tian, W. (2013). An inductive signalling network regulates mammalian tooth morphogenesis with implications for tooth regeneration. Cell Proliferation, 46(5), 501–508. Graf, D., Malik, Z., Hayano, S., & Mishina, Y. (2016). Common mechanisms in development and disease: BMP signaling in craniofacial development. Cytokine & Growth Factor Reviews, 27, 129–139. Chai, Y., & Maxson, R. (2006). Recent advances in craniofacial morphogenesis. Developmental Dynamics, 235(9), 2353–2375. Laurikkala, J., Mikkola, M., Mustonen, T., Aberg, T., Koppinen, P., Pispa, J., Nieminen, P., Galceran, J., Grosschedl, R., & Thesleff, I. (2001). TNF signaling via the ligand-receptor pair ectodysplasin and edar controls the function of epithelial signaling centers and is regulated by Wnt and activin during tooth organogenesis. Developmental Biology, 229(2), 443–455. https://doi.org/10.1006/dbio.2000.9955. Wang, J., & Martin, J. F. (2017). Hippo pathway: An emerging regulator of craniofacial and dental development. Journal of Dental Research, 96(11), 1229–1237. https://doi.org/10.1177/0022034517719886. **e, F., Dai, Q., Liu, X., & Wang, J. (2019). Conditional knockout of raptor/mTORC1 results in dentin malformation. Frontiers in Physiology, 10, 250. https://doi.org/10.3389/fphys.2019.00250. Jia, S., Zhou, J., Gao, Y., Baek, J., Martin, J., Lan, Y., & Jiang, R. (2013). Roles of Bmp4 during tooth morphogenesis and sequential tooth formation. Development, 140(2), 423–432. Jia, S., Kwon, H., Lan, Y., Zhou, J., Liu, H., & Jiang, R. (2016). Bmp4-Msx1 signaling and Osr2 control tooth organogenesis through antagonistic regulation of secreted Wnt antagonists. Developmental Biology, 420(1), 110–119. O'Connell, D., Ho, J., Mammoto, T., Turbe-Doan, A., O'Connell, J., Haseley, P., . . . Maas, R. (2012). A Wnt-bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Sci Signal, 5(206), ra4. Gao, Z., Wang, L., Wang, F., Zhang, C., Wang, J., He, J., & Wang, S. (2018). Expression of BMP2/4/7 during the odontogenesis of deciduous molars in miniature pig embryos. Journal of Molecular Histology. Malik, Z., Alexiou, M., Hallgrimsson, B., Economides, A., Luder, H., & Graf, D. (2018). Bone morphogenetic protein 2 coordinates early tooth mineralization. Journal of Dental Research, 97(7), 835–843. Huang, X., Wang, F., Zhao, C., Yang, S., Cheng, Q., Tang, Y., Zhang, F., Zhang, Y., Luo, W., Wang, C., Zhou, P., Kim, S., Zuo, G., Hu, N., Li, R., He, T. C., & Zhang, H. (2019). Dentinogenesis and tooth-alveolar bone complex defects in BMP9/GDF2 knockout mice. Stem Cells and Development, 28(10), 683–694. https://doi.org/10.1089/scd.2018.0230. Kettunen, P., & Thesleff, I. (1998). Expression and function of FGFs-4, −8, and −9 suggest functional redundancy and repetitive use as epithelial signals during tooth morphogenesis. Developmental Dynamics, 211(3), 256–268. Kettunen, P., Laurikkala, J., Itäranta, P., Vainio, S., Itoh, N., & Thesleff, I. (2000). Associations of FGF-3 and FGF-10 with signaling networks regulating tooth morphogenesis. Developmental Dynamics, 219(3), 322–332. Porntaveetus, T., Otsuka-Tanaka, Y., Basson, M., Moon, A., Sharpe, P., & Ohazama, A. (2011). Expression of fibroblast growth factors (Fgfs) in murine tooth development. Journal of Anatomy, 218(5), 534–543. Neubüser, A., Peters, H., Balling, R., & Martin, G. (1997). Antagonistic interactions between FGF and BMP signaling pathways: A mechanism for positioning the sites of tooth formation. Cell, 90(2), 247–255. Prochazka, J., Prochazkova, M., Du, W., Spoutil, F., Tureckova, J., Hoch, R., . . . Klein, O. (2015). Migration of founder epithelial cells drives proper molar tooth positioning and morphogenesis. Developmental Cell, 35(6), 713–724. Zhou, C., Yang, G., Chen, M., He, L., **ang, L., Ricupero, C., Mao, J. J., & Ling, J. (2015). Lhx6 and Lhx8: Cell fate regulators and beyond. The FASEB Journal, 29(10), 4083–4091. Zhou, C., Yang, G., Chen, M., Wang, C., He, L., **ang, L., Chen, D., Ling, J., & Mao, J. (2015). Lhx8 mediated Wnt and TGFβ pathways in tooth development and regeneration. Biomaterials, 63, 35–46. Tai, Y. Y., Chen, R. S., Lin, Y., Ling, T. Y., & Chen, M. H. (2012). FGF-9 accelerates epithelial invagination for ectodermal organogenesis in real time bioengineered organ manipulation. Cell Communication and Signaling: CCS, 10(1), 34. https://doi.org/10.1186/1478-811x-10-34. Wang, B., Li, H., Liu, Y., Lin, X., Lin, Y., Wang, Y., Hu, X., & Zhang, Y. (2014). Expression patterns of WNT/β-CATENIN signaling molecules during human tooth development. Journal of Molecular Histology, 45(5), 487–496. Sarkar, L., Cobourne, M., Naylor, S., Smalley, M., Dale, T., & Sharpe, P. T. (2000). Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proceedings of the National Academy of Sciences of the United States of America, 97(9), 4520–4524. https://doi.org/10.1073/pnas.97.9.4520. Dassule, H., & McMahon, A. (1998). Analysis of epithelial-mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Developmental Biology, 202(2), 215–227. Sarkar, L., & Sharpe, P. T. (1999). Expression of Wnt signalling pathway genes during tooth development. Mechanisms of Development, 85(1–2), 197–200. Wu, X., Li, Y., Wang, F., Hu, L., Li, Y., Wang, J., Zhang, C., & Wang, S. (2017). Spatiotemporal expression of Wnt/β-catenin signaling during morphogenesis and Odontogenesis of deciduous molar in miniature pig. International Journal of Biological Sciences, 13(8), 1082–1091. van Genderen, C., Okamura, R., Fariñas, I., Quo, R., Parslow, T., Bruhn, L., & Grosschedl, R. (1994). Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes & Development, 8(22), 2691–2703. Bae, C., Kim, T., Ko, S., Lee, J., Yang, X., & Cho, E. (2015). Wntless regulates dentin apposition and root elongation in the mandibular molar. Journal of Dental Research, 94(3), 439–445. Aurrekoetxea, M., Irastorza, I., García-Gallastegui, P., Jiménez-Rojo, L., Nakamura, T., Yamada, Y., . . . Unda, F. (2016). Wnt/β-catenin regulates the activity of Epiprofin/Sp6, SHH, FGF, and BMP to coordinate the stages of Odontogenesis. Frontiers in Cell and Development Biology, 4, 25. Yu, P., Yang, W., Han, D., Wang, X., Guo, S., Li, J., Li, F., Zhang, X., Wong, S. W., Bai, B., Liu, Y., du, J., Sun, Z. S., Shi, S., Feng, H., & Cai, T. (2016). Mutations in WNT10B are identified in individuals with Oligodontia. American Journal of Human Genetics, 99(1), 195–201. Johnson, R. L., & Tabin, C. (1995). The long and short hedgehog signaling. 81(3), 313-316. Vaahtokari, A., Åberg, T., Jernvall, J., Keränen, S., & Thesleff, I. (1996). The enamel knot as a signaling center in the develo** mouse tooth. Mechanisms of Development, 54(1), 39–43. Koyama, E., Yamaai, T., Iseki, S., Ohuchi, H., Nohno, T., Yoshioka, H., . . . Noji, S. (1996). Polarizing activity, sonic hedgehog, and tooth development in embryonic and postnatal mouse. Developmental Dynamics, 206(1), 59–72. Dassule, H., Lewis, P., Bei, M., Maas, R., & McMahon, A. (2000). Sonic hedgehog regulates growth and morphogenesis of the tooth. Development, 127(22), 4775–4785. Bae, W. J., Auh, Q. S., Lim, H. C., Kim, G. T., Kim, H. S., & Kim, E. C. (2016). Sonic hedgehog promotes Cementoblastic differentiation via activating the BMP pathways. Calcified Tissue International, 99(4), 396–407. Li, J., Feng, J., Liu, Y., Ho, T., Grimes, W., Ho, H., Park, S., Wang, S., & Chai, Y. (2015). BMP-SHH signaling network controls epithelial stem cell fate via regulation of its niche in the develo** tooth. Developmental Cell, 33(2), 125–135. Cho, S. W., Kwak, S., Woolley, T. E., Lee, M. J., Kim, E. J., Baker, R. E., . . . Maini, P. K. (2011). Interactions between Shh, Sostdc1 and Wnt signaling and a new feedback loop for spatial patterning of the teeth. Development, 138(9), 1807–1816. Kowalczyk-Quintas, C., & Schneider, P. (2014). Ectodysplasin a (EDA) - EDA receptor signalling and its pharmacological modulation. Cytokine & Growth Factor Reviews, 25(2), 195–203. Pispa, J., Jung, H., Jernvall, J., Kettunen, P., Mustonen, T., Tabata, M., Kere, J., & Thesleff, I. (1999). Cusp patterning defect in Tabby mouse teeth and its partial rescue by FGF. Developmental Biology, 216(2), 521–534. Shen, W., Wang, Y., Liu, Y., Liu, H., Zhao, H., Zhang, G., Snead, M. L., Han, D., & Feng, H. (2016). Functional study of Ectodysplasin-a mutations causing non-Syndromic tooth agenesis. PLoS One, 11(5), e0154884. Fahmy, R., Mahmoud, N., Soliman, S., Nouh, S., Cunningham, L., & El-Ghannam, A. (2015). Acceleration of alveolar ridge augmentation using a low dose of recombinant human bone morphogenetic Protein-2 loaded on a Resorbable bioactive ceramic. Journal of Oral and Maxillofacial Surgery, 73(12), 2257–2272. Zhu, S., Nih, L., Carmichael, S., Lu, Y., & Segura, T. (2015). Enzyme-responsive delivery of multiple proteins with spatiotemporal control. Adv. Mater. Weinheim, 27(24), 3620–3625. Knaack, S., Lode, A., Hoyer, B., Rösen-Wolff, A., Gabrielyan, A., Roeder, I., & Gelinsky, M. (2014). Heparin modification of a biomimetic bone matrix for controlled release of VEGF. Journal of Biomedical Materials Research. Part A, 102(10), 3500–3511. Wu, J., Ye, J., Zhu, J., **ao, Z., He, C., Shi, H., Wang, Y., Lin, C., Zhang, H., Zhao, Y., Fu, X., Chen, H., Li, X., Li, L., Zheng, J., & **ao, J. (2016). Heparin-based Coacervate of FGF2 improves dermal regeneration by asserting a synergistic role with cell proliferation and endogenous facilitated VEGF for cutaneous wound healing. Biomacromolecules, 17(6), 2168–2177. Shah, N., Hyder, M., Quadir, M., Dorval Courchesne, N., Seeherman, H., Nevins, M., Spector, M., & Hammond, P. (2014). Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proceedings of the National Academy of Sciences of the United States of America, 111(35), 12847–12852. Mei, L., Wang, Y., Tong, A., & Guo, G. (2016). Facile electrospinning of an efficient drug delivery system. Expert Opinion on Drug Delivery, 13(5), 741–753. Kocak, G., Tuncer, C., & Bütün, V. (2016). pH-responsive polymers. Polymer Chemistry, 8(1), 144–176. Baliga, S., Muglikar, S., & Kale, R. (2013). Salivary pH: A diagnostic biomarker. Journal of Indian Society of Periodontology, 17(4), 461–465. Kalhapure, R. S., Jadhav, M., Rambharose, S., Mocktar, C., Singh, S., Renukuntla, J., & Govender, T. (2017). pH-responsive chitosan nanoparticles from a novel twin-chain anionic amphiphile for controlled and targeted delivery of vancomycin. Colloids & Surfaces B Biointerfaces, 158(4), 650. Halacheva, S. S., Adlam, D. J., Hendow, E. K., Freemont, T. J., Hoyland, J., & Saunders, B. R. (2014). Injectable biocompatible and biodegradable pH-responsive hollow particle gels containing poly(acrylic acid): The effect of copolymer composition on gel properties. Biomacromolecules, 15(5), 1814–1827. Echazú, M. I. A., Olivetti, C. E., Peralta, I., Alonso, M. R., Anesini, C., Perez, C. J., . . . Desimone, M. F. (2018). Development of pH-responsive biopolymer-silica composites loaded with Larrea divaricata Cav. Extract with antioxidant activity. Colloids and Surfaces. B, Biointerfaces, 169, 82–91. Sensenig, R., Sapir, Y., Macdonald, C., Cohen, S., & Polyak, B. (2012). Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine, 7(9), 1425–1442. Li, Y., Ye, D., Li, M., Ma, M., & Gu, N. (2018). Adaptive materials based on Iron oxide nanoparticles for bone regeneration. Chemphyschem A European Journal of Chemical Physics & Physical Chemistry. Fan, M., Yan, J., Tan, H., Miao, Y., & Hu, X. (2014). Magnetic biopolymer nanogels via biological assembly for vectoring delivery of biopharmaceuticals. Journal of Materials Chemistry B, 2(47), 8399–8405. Karahaliloğlu, Z., Yalçın, E., Demirbilek, M., & Denkbaş, E. B. (2017). Magnetic silk fibroin e-gel scaffolds for bone tissue engineering applications. Journal of Bioactive\s&\scompatible Polymers, 32(6), 088391151769363. O'Brien, C. M., Holmes, B., Faucett, S., & Zhang, L. G. (2015). Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Engineering. Part B, Reviews, 21(1), 103–114. Legemate, K., Tarafder, S., Jun, Y., & Lee, C. (2016). Engineering human TMJ discs with protein-releasing 3D-printed scaffolds. Journal of Dental Research, 95(7), 800–807. Hamlet, S. M., Vaquette, C., Shah, A., Hutmacher, D. W., & Ivanovski, S. (2016). 3-dimensional functionalized polycaprolactone-hyaluronic acid hydrogel constructs for bone tissue engineering. Journal of Clinical Periodontology, 44(4), 428. Huang, K., Lin, Y., Shie, M., & Lin, C. (2018). Effects of bone morphogenic protein-2 loaded on the 3D-printed MesoCS scaffolds. Journal of the Formosan Medical Association, 117(10), 879–887. Wang, C., Zhao, Q., & Wang, M. (2017). Cryogenic 3D printing for producing hierarchical porous and rhBMP-2-loaded Ca-P/PLLA nanocomposite scaffolds for bone tissue engineering. Biofabrication, 9(2), 025031. Li, S., Xu, Y., Yu, J., & Becker, M. L. (2017). Enhanced osteogenic activity of poly(ester urea) scaffolds using facile post-3D printing peptide functionalization strategies. Biomaterials, 141, 176–187. Wagner, E. R., Parry, J., Dadsetan, M., Bravo, D., Riester, S. M., Wijnen, A. J. V., . . . Kakar, S. (2018). VEGF-mediated angiogenesis and vascularization of a fumarate-crosslinked polycaprolactone (PCLF) scaffold. Connective Tissue Research, 1-8. **ong, S., Zhang, X., Lu, P., Wu, Y., Wang, Q., Sun, H., Heng, B. C., Bunpetch, V., Zhang, S., & Ouyang, H. (2017). A gelatin-sulfonated silk composite scaffold based on 3D printing technology enhances skin regeneration by stimulating epidermal growth and dermal neovascularization. Scientific Reports, 7(1), 4288. Miao, S., Castro, N., Nowicki, M., **a, L., Cui, H., Zhou, X., . . . Vozzi, G. (2017). 4D printing of polymeric materials for tissue and organ regeneration. Materials Today, 20(10), 577, 591. Fire, A., Xu, S., Montgomery, M. K., Kostas, S. A., Driver, S. E., & Mello, C. C. (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature, 391(6669), 806–811. https://doi.org/10.1038/35888. Kanasty, R., Dorkin, J. R., Vegas, A., & Anderson, D. (2013). Delivery materials for siRNA therapeutics. Nature Materials, 12(11), 967–977. https://doi.org/10.1038/nmat3765. Zhang, G., Guo, B., Wu, H., Tang, T., Zhang, B. T., Zheng, L., He, Y., Yang, Z., Pan, X., Chow, H., To, K., Li, Y., Li, D., Wang, X., Wang, Y., Lee, K., Hou, Z., Dong, N., Li, G., Leung, K., Hung, L., He, F., Zhang, L., & Qin, L. (2012). A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nature Medicine, 18(2), 307–314. https://doi.org/10.1038/nm.2617. Castleberry, S. A., Almquist, B. D., Li, W., Reis, T., Chow, J., Mayner, S., & Hammond, P. T. (2016). Self-assembled wound dressings silence MMP-9 and improve diabetic wound healing in vivo. Advanced Materials, 28(9), 1809–1817. https://doi.org/10.1002/adma.201503565. Wittrup, A., & Lieberman, J. (2015). Knocking down disease: A progress report on siRNA therapeutics. Nature Reviews. Genetics, 16(9), 543–552. https://doi.org/10.1038/nrg3978. Lim, L. P., Lau, N. C., Garrett-Engele, P., Grimson, A., Schelter, J. M., Castle, J., Bartel, D. P., Linsley, P. S., & Johnson, J. M. (2005). Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature, 433(7027), 769–773. https://doi.org/10.1038/nature03315. Bartel, D. P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell, 116(2), 281–297. https://doi.org/10.1016/s0092-8674(04)00045-5. Li, D., Wang, A., Liu, X., Meisgen, F., Grunler, J., Botusan, I. R., . . . Landen, N. X. (2015). MicroRNA-132 enhances transition from inflammation to proliferation during wound healing. The Journal of Clinical Investigation, 125(8), 3008–3026. doi:https://doi.org/10.1172/jci79052. Yang, Y., Cheng, H. W., Qiu, Y., Dupee, D., Noonan, M., Lin, Y. D., Fisch, S., Unno, K., Sereti, K. I., & Liao, R. (2015). MicroRNA-34a plays a key role in cardiac repair and regeneration following myocardial infarction. Circulation Research, 117(5), 450–459. https://doi.org/10.1161/circresaha.117.305962. Eulalio, A., Mano, M., Dal Ferro, M., Zentilin, L., Sinagra, G., Zacchigna, S., & Giacca, M. (2012). Functional screening identifies miRNAs inducing cardiac regeneration. Nature, 492(7429), 376–381. https://doi.org/10.1038/nature11739. Aguirre, A., Montserrat, N., Zacchigna, S., Nivet, E., Hishida, T., Krause, M. N., Kurian L., Ocampo A., Vazquez-Ferrer E., Rodriguez-Esteban C., Kumar S., Moresco J.J., Yates JR 3rd, Campistol J.M., Sancho-Martinez I., Giacca M. Izpisua Belmonte, J. C. (2014). In vivo activation of a conserved microRNA program induces mammalian heart regeneration. Cell Stem Cell, 15(5), 589–604. https://doi.org/10.1016/j.stem.2014.10.003. Zhang, X., Li, Y., Chen, Y. E., Chen, J., & Ma, P. X. (2016). Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nature Communications, 7, 10376. https://doi.org/10.1038/ncomms10376. Zhou, F., Jia, X., Yang, Y., Yang, Q., Gao, C., Hu, S., Zhao, Y., Fan, Y., & Yuan, X. (2016). Nanofiber-mediated microRNA-126 delivery to vascular endothelial cells for blood vessel regeneration. Acta Biomaterialia, 43, 303–313. https://doi.org/10.1016/j.actbio.2016.07.048. Lesizza, P., Prosdocimo, G., Martinelli, V., Sinagra, G., Zacchigna, S., & Giacca, M. (2017). Single-dose Intracardiac injection of pro-regenerative MicroRNAs improves cardiac function after myocardial infarction. Circulation Research, 120(8), 1298–1304. https://doi.org/10.1161/circresaha.116.309589. Pyo, K. H., Lim, S. M., Kim, H. R., Sung, Y. H., Yun, M. R., Kim, S. M., Kim, H., Kang, H. N., Lee, J. M., Kim, S. G., Park, C. W., Chang, H., Shim, H. S., Lee, H. W., & Cho, B. C. (2017). Establishment of a conditional transgenic mouse model recapitulating EML4-ALK-positive human non-small cell lung Cancer. Journal of Thoracic Oncology, 12(3), 491–500. https://doi.org/10.1016/j.jtho.2016.10.022. Kanisicak, O., Khalil, H., Ivey, M. J., Karch, J., Maliken, B. D., Correll, R. N., Brody, M. J., J Lin, S. C., Aronow, B. J., Tallquist, M. D., & Molkentin, J. D. (2016). Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nature Communications, 7, 12260. https://doi.org/10.1038/ncomms12260. Molkentin, J. D., Bugg, D., Ghearing, N., Dorn, L. E., Kim, P., Sargent, M. A., Gunaje, J., Otsu, K., & Davis, J. (2017). Fibroblast-specific genetic manipulation of p38 mitogen-activated protein kinase in vivo reveals its central regulatory role in fibrosis. Circulation, 136(6), 549–561. https://doi.org/10.1161/circulationaha.116.026238. Senturk, S., Shirole, N. H., Nowak, D. G., Corbo, V., Pal, D., Vaughan, A., Tuveson, D. A., Trotman, L. C., Kinney, J. B., & Sordella, R. (2017). Rapid and tunable method to temporally control gene editing based on conditional Cas9 stabilization. Nature Communications, 8, 14370. https://doi.org/10.1038/ncomms14370. Park, S., Koppes, R. A., Froriep, U. P., Jia, X., Achyuta, A. K. H., Mclaughlin, B. L., & Anikeeva, P. (2015). Optogenetic control of nerve growth. Scientific Reports, 5, 9669. Kim, N., Kim, J. M., Lee, M., Kim, C. Y., Chang, K. Y., & Heo, W. D. (2014). Spatiotemporal control of fibroblast growth factor receptor signals by blue light. Chemistry & Biology, 21(7), 903–912. https://doi.org/10.1016/j.chembiol.2014.05.013. Bugaj, L. J., Choksi, A. T., Mesuda, C. K., Kane, R. S., & Schaffer, D. V. (2013). Optogenetic protein clustering and signaling activation in mammalian cells. Nature Methods, 10(3), 249–252. Li, Y., Lee, M., Kim, N., Wu, G., Deng, D., Kim, J., Liu, X., Heo, W. D., & Zi, Z. (2018). Spatiotemporal control of TGF-β signaling with light. ACS Synthetic Biology, 7(2), 443–451. Beyer, H. M., Naumann, S., Weber, W., & Radziwill, G. (2015). Optogenetic control of signaling in mammalian cells. Biotechnology Journal, 10(2), 273–283. Wang, X., Chen, X., & Yang, Y. (2012). Spatiotemporal control of gene expression by a light-switchable transgene system. Nature Methods, 9(3), 266–269. Distel, M., Wullimann, M. F., & Köster, R. W. (2009). Optimized Gal4 genetics for permanent gene expression map** in zebrafish. Proceedings of the National Academy of Sciences of the United States of America, 106(32), 13365–13370. Mottamena, L. B., Reade, A., Mallory, M. J., Glantz, S., Weiner, O. D., Lynch, K. W., & Gardner, K. H. (2014). An optogenetic gene expression system with rapid activation and deactivation kinetics. Nature Chemical Biology, 10(3), 196–202. Reade, A., Motta-Mena, L. B., Gardner, K. H., Stainier, D. Y., Weiner, O. D., & Woo, S. (2017). TAEL: A zebrafish-optimized optogenetic gene expression system with fine spatial and temporal control. Development, 144(2), 345–355. Schindler, S. E., Mccall, J. G., **, Y., Hyrc, K. L., Li, M., Tucker, C. L., . . . Diamond, M. I. (2015). Photo-activatable Cre recombinase regulates gene expression in vivo. Scientific Reports, 5, 13627. Kawano, F., Okazaki, R., Yazawa, M., & Sato, M. (2016). A photoactivatable Cre-loxP recombination system for optogenetic genome engineering. Nature Chemical Biology, 12(12), 1059–1064. Nguyen, N., He, L., Martinez-Moczygemba, M., Huang, Y., & Zhou, Y. (2018). Rewiring calcium signaling for precise transcriptional reprogramming. ACS Synthetic Biology, 7(3), 814–821. Shao, J., Wang, M., Yu, G., Zhu, S., Yu, Y., Heng, B., Wu, J., & Ye, H. (2018). Synthetic far-red light-mediated CRISPR-dCas9 device for inducing functional neuronal differentiation. Proceedings of the National Academy of Sciences of the United States of America, 115(29), E6722–E6730. Chai, Y., Jiang, X., Ito, Y., Bringas, P., Jr., Han, J., Rowitch, D. H., . . . Sucov, H. M. (2000). Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development, 127(8), 1671–1679. Liu, B., Chen, S., Cheng, D., **g, W., & Helms, J. A. (2014). Primary cilia integrate hedgehog and Wnt signaling during tooth development. Journal of Dental Research, 93(5), 475–482. https://doi.org/10.1177/0022034514528211. Guo, S., Zhang, Y., Zhou, T., Wang, D., Weng, Y., Wang, L., & Ma, J. (2017). Role of GATA binding protein 4 (GATA4) in the regulation of tooth development via GNAI3. Scientific Reports, 7(1), 1534. https://doi.org/10.1038/s41598-017-01689-1. Chen, H., Guo, S., **a, Y., Yuan, L., Lu, M., Zhou, M., Fang, M., Meng, L., **ao, Z., & Ma, J. (2018). The role of rho-GEF trio in regulating tooth root development through the p38 MAPK pathway. Experimental Cell Research, 372(2), 158–167. https://doi.org/10.1016/j.yexcr.2018.09.022. Jacob, A., Zhang, Y., & George, A. (2014). Transcriptional regulation of dentin matrix protein 1 (DMP1) in odontoblasts and osteoblasts. Connective Tissue Research, 55(Suppl 1), 107–112. https://doi.org/10.3109/03008207.2014.923850. Kotterman, M. A., Chalberg, T. W., & Schaffer, D. V. (2015). Viral vectors for gene therapy: Translational and clinical outlook. Annual Review of Biomedical Engineering, 17, 63–89. https://doi.org/10.1146/annurev-bioeng-071813-104938. Eggers, R., de Winter, F., Hoyng, S. A., Roet, K. C., Ehlert, E. M., Malessy, M. J., Verhaagen, J., & Tannemaat, M. R. (2013). Lentiviral vector-mediated gradients of GDNF in the injured peripheral nerve: Effects on nerve coil formation, Schwann cell maturation and myelination. PLoS One, 8(8), e71076. https://doi.org/10.1371/journal.pone.0071076. Li, Y. H., Zhang, K., Yang, K., Ye, J. X., **ng, Y. Z., Guo, H. Y., et al. (2013). Adenovirus-mediated Wnt10b overexpression induces hair follicle regeneration. The Journal of Investigative Dermatology, 133(1), 42–48. https://doi.org/10.1038/jid.2012.235. Hu, W. W., Wang, Z., & Krebsbach, P. H. (2016). Virus immobilization on biomaterial scaffolds through biotin-avidin interaction for improving bone regeneration. Journal of Tissue Engineering and Regenerative Medicine, 10(2), E63–E72. https://doi.org/10.1002/term.1774. Dalsgaard, T., Cecchi, C. R., Askou, A. L., Bak, R. O., Andersen, P. O., Hougaard, D., Jensen, T. G., Dagnæs-Hansen, F., Mikkelsen, J. G., Corydon, T. J., & Aagaard, L. (2018). Improved Lentiviral gene delivery to mouse liver by hydrodynamic vector injection through tail vein. Mol Ther Nucleic Acids, 12, 672–683. https://doi.org/10.1016/j.omtn.2018.07.005. Yin, H., Kanasty, R. L., Eltoukhy, A. A., Vegas, A. J., Dorkin, J. R., & Anderson, D. G. (2014). Non-viral vectors for gene-based therapy. Nature Reviews. Genetics, 15(8), 541–555. https://doi.org/10.1038/nrg3763. Mun, J. Y., Shin, K. K., Kwon, O., Yong, T. L., & Oh, D. B. (2016). Minicircle microporation-based non-viral gene delivery improved the targeting of mesenchymal stem cells to an injury site. Biomaterials, 101, 310–320. Molla, M. R., & Levkin, P. A. (2016). Combinatorial approach to Nanoarchitectonics for nonviral delivery of nucleic acids. Advanced Materials, 28(6), 1159–1175. https://doi.org/10.1002/adma.201502888. Curtin, C. M., Tierney, E. G., Mcsorley, K., Cryan, S. A., Duffy, G. P., & O'Brien, F. J. (2015). Combinatorial gene therapy accelerates bone regeneration: Non-viral dual delivery of VEGF and BMP2 in a collagen-nanohydroxyapatite scaffold. Advanced Healthcare Materials, 4(2), 223. Mout, R., Ray, M., Yesilbag Tonga, G., Lee, Y. W., Tay, T., Sasaki, K., & Rotello, V. M. (2017). Direct cytosolic delivery of CRISPR/Cas9-Ribonucleoprotein for efficient gene editing. ACS Nano, 11(3), 2452–2458. https://doi.org/10.1021/acsnano.6b07600. **ang, D., Liu, C. C., Wang, M. J., Li, J. X., Chen, F., Yao, H., Yu, B., Lu, L., Borjigin, U., Chen, Y. X., Zhong, L., Wangensteen, K. J., He, Z. Y., Wang, X., & Hu, Y. P. (2014). Non-viral FoxM1 gene delivery to hepatocytes enhances liver repopulation. Cell Death & Disease, 5, e1252. https://doi.org/10.1038/cddis.2014.230. Lee, Y. H., Wu, H. C., Yeh, C. W., Kuan, C. H., Liao, H. T., Hsu, H. C., Tsai, J. C., Sun, J. S., & Wang, T. W. (2017). Enzyme-crosslinked gene-activated matrix for the induction of mesenchymal stem cells in osteochondral tissue regeneration. Acta Biomaterialia, 63, 210–226. https://doi.org/10.1016/j.actbio.2017.09.008. Yang, Q. Q., Shao, Y. X., Zhang, L. Z., & Zhou, Y. L. (2018). Therapeutic strategies for flexor tendon healing by nanoparticle-mediated co-delivery of bFGF and VEGFA genes. Colloids and Surfaces. B, Biointerfaces, 164, 165–176. https://doi.org/10.1016/j.colsurfb.2018.01.031. Zhang, N., Chin, J. S., & Chew, S. Y. (2019). Localised non-viral delivery of nucleic acids for nerve regeneration in injured nervous systems. Experimental Neurology, 319, 112820. https://doi.org/10.1016/j.expneurol.2018.09.003. Feng, G., Zhang, Z., Dang, M., Zhang, X., Doleyres, Y., Song, Y., Chen, D., & Ma, P. X. (2017). Injectable nanofibrous spongy microspheres for NR4A1 plasmid DNA transfection to reverse fibrotic degeneration and support disc regeneration. Biomaterials, 131, 86–97. https://doi.org/10.1016/j.biomaterials.2017.03.029. Azoidis, I., Cox, S. C., & Davies, O. G. (2018). The role of extracellular vesicles in biomineralisation: Current perspective and application in regenerative medicine. J Tissue Eng, 9, 2041731418810130. https://doi.org/10.1177/2041731418810130. Boorn, J. G., Den, V., Martin, S., Christoph, C., & Gunther, H. (2011). SiRNA delivery with exosome nanoparticles. Nature Biotechnology, 29(4), 325–326. Rani, S., & Ritter, T. (2016). The exosome - a naturally secreted nanoparticle and its application to wound healing. Advanced Materials, 28(27), 5542–5552. https://doi.org/10.1002/adma.201504009. Elangovan, S., Khorsand, B., Do, A. V., Hong, L., Dewerth, A., Kormann, M., Ross, R. D., Sumner, D. R., Allamargot, C., & Salem, A. K. (2015). Chemically modified RNA activated matrices enhance bone regeneration. Journal of Controlled Release, 218, 22–28. https://doi.org/10.1016/j.jconrel.2015.09.050. Khorsand, B., Elangovan, S., Hong, L., Dewerth, A., Kormann, M. S., & Salem, A. K. (2017). A comparative study of the bone regenerative effect of chemically modified RNA encoding BMP-2 or BMP-9. The AAPS Journal, 19(2), 438–446. https://doi.org/10.1208/s12248-016-0034-8. Mavilio, F., Pellegrini, G., Ferrari, S., Nunzio, F. D., Iorio, E. D., Recchia, A., . . . Bonini, C. (2006). Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nature Medicine, 12(12), 1397–1402. Lu, Y. C., Parker, L. L., Lu, T., Zheng, Z., Toomey, M. A., White, D. E., . . . Feldman, S. A. (2017). Treatment of patients with metastatic Cancer using a major histocompatibility complex class II-restricted T-cell receptor targeting the Cancer Germline antigen MAGE-A3. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology, 35(29), 3322, 3329. Gerace, D., Martiniellowilks, R., Nassif, N. T., Lal, S., Steptoe, R., & Simpson, A. M. (2017). CRISPR-targeted genome editing of mesenchymal stem cell-derived therapies for type 1 diabetes: A path to clinical success? Stem Cell Research & Therapy, 8(1), 62. Evans, C. H., & Huard, J. (2015). Gene therapy approaches to regenerating the musculoskeletal system. Nature Reviews Rheumatology, 11(4), 234–242. Hamada, H., Kobune, M., Nakamura, K., Kawano, Y., Kato, K., Honmou, O., Houkin, K., Matsunaga, T., & Niitsu, Y. (2005). Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy. Cancer Science, 96(3), 149–156. https://doi.org/10.1111/j.1349-7006.2005.00032.x. Liu, J., Chen, W., Zhao, Z., & Xu, H. H. (2013). Reprogramming of mesenchymal stem cells derived from iPSCs seeded on biofunctionalized calcium phosphate scaffold for bone engineering. Biomaterials, 34(32), 7862–7872. Zhang, F., Peng, W. X., Wang, L., Zhang, J., Dong, W. T., Wu, J. H., Zhang, H., Wang, J. B., & Zhao, Y. (2018). Role of FGF-2 transfected bone marrow Mesenchymal stem cells in engineered bone tissue for repair of avascular necrosis of femoral head in rabbits. Cellular Physiology and Biochemistry, 48(2), 773–784. https://doi.org/10.1159/000491906. Ankrum, J. A., Ong, J. F., & Karp, J. M. (2014). Mesenchymal stem cells: immune evasive, not immune privileged. Nature Biotechnology, 32(3), 252–260. https://doi.org/10.1038/nbt.2816. Terai, S., & Tsuchiya, A. (2016). Status of and candidates for cell therapy in liver cirrhosis: Overcoming the "point of no return" in advanced liver cirrhosis. Journal of Gastroenterology, 52(2), 1–12. Ben-Mordechai, T., Holbova, R., Landa-Rouben, N., Harel-Adar, T., Feinberg, M. S., Abd Elrahman, I., Blum, G., Epstein, F. H., Silman, Z., Cohen, S., & Leor, J. (2013). Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. Journal of the American College of Cardiology, 62(20), 1890–1901. https://doi.org/10.1016/j.jacc.2013.07.057. Spiller, K. L., & Koh, T. J. (2017). Macrophage-based therapeutic strategies in regenerative medicine. Advanced Drug Delivery Reviews.
Thomas, J. A., Pope, C., Wojtacha, D., Robson, A. J., Gordon-Walker, T. T., Hartland, S., . . . Iredale, J. P. (2011). Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function &dagger. Hepatology, 53(6), 2003–2015. Boulter, L., Govaere, O., Bird, T. G., Radulescu, S., Ramachandran, P., Pellicoro, A., Ridgway, R. A., Seo, S. S., Spee, B., van Rooijen, N., Sansom, O. J., Iredale, J. P., Lowell, S., Roskams, T., & Forbes, S. J. (2012). Macrophage-derived Wnt opposes notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nature Medicine, 18(4), 572–579. https://doi.org/10.1038/nm.2667. Hamm, A., Veschini, L., Takeda, Y., Costa, S., Delamarre, E., Squadrito, M. L., Henze, A. T., Wenes, M., Serneels, J., Pucci, F., Roncal, C., Anisimov, A., Alitalo, K., de Palma, M., & Mazzone, M. (2013). PHD2 regulates arteriogenic macrophages through TIE2 signalling. EMBO Molecular Medicine, 5(6), 843–857. https://doi.org/10.1002/emmm.201302695. Nakashima, M., & Reddi, A. H. (2003). The application of bone morphogenetic proteins to dental tissue engineering. Nature Biotechnology, 21(9), 1025–1032. Han, P., Ivanovski, S., Crawford, R., & **ao, Y. (2015). Activation of the canonical Wnt signaling pathway induces Cementum regeneration. Journal of Bone and Mineral Research, 30(7), 1160–1174. https://doi.org/10.1002/jbmr.2445. Nakao, K., Morita, R., Saji, Y., Ishida, K., Tomita, Y., Ogawa, M., Saitoh, M., Tomooka, Y., & Tsuji, T. (2007). The development of a bioengineered organ germ method. Nature Methods, 4(3), 227–230. https://doi.org/10.1038/nmeth1012. Ikeda, E., Morita, R., Nakao, K., Ishida, K., Nakamura, T., Takano-Yamamoto, T., Ogawa, M., Mizuno, M., Kasugai, S., & Tsuji, T. (2009). Fully functional bioengineered tooth replacement as an organ replacement therapy. Proceedings of the National Academy of Sciences of the United States of America, 106(32), 13475–13480. https://doi.org/10.1073/pnas.0902944106.References
Acknowledgements
Funding: This work was supported by grants from National Key R&D Program of China (2016YFC20160905200), National Natural Science Foundation of China (81600824), Natural Science Foundation of Guangdong Province (2016A030310220, 2018A030310278), Young Teachers Training Program of Sun Yat-sen University (16ykpy46, 17ykpy73), Science and Technology Program of Guangzhou (201707010106, 201804010459) and the Young Elite Scientist Sponsorship Program by CAST (2016QNRC001).
Author information
Authors and Affiliations
Contributions
DH was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, D., Ren, J., Li, R. et al. Tooth Regeneration: Insights from Tooth Development and Spatial-Temporal Control of Bioactive Drug Release. Stem Cell Rev and Rep 16, 41–55 (2020). https://doi.org/10.1007/s12015-019-09940-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-019-09940-0