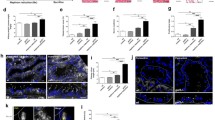
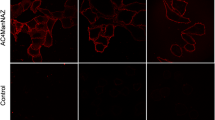
Abstract
During mitosis, phosphorylation and dephosphorylation of lamins triggers the nuclear envelope disassembly/assembly. However, it hasn’t been known whether lamin proteins undergo any modification other than phosphorylation during the cell cycle. Glycosylation of lamin proteins is one of the less studied post-translational modification. Glycosylation and phosphorylation compete for the same positions and interplay between two modifications generate a post-translational code in the cell. Based on this, we hypothesized that glycosylation of lamin A/C protein may be important in the regulation of the structural organization of the nuclear lamina during interphase and mitosis. We analysed the glycan units of lamin A/C protein in lung carcinoma cells synchronized at G2/M and S phases via CapLC-ESI-MS/MS. Besides, the outermost glycan units were determined using lectin blotting and gold-conjugated antibody and lectin staining. TEM studies also allowed us to observe the localization of glycosylated lamin A/C protein. With this study, we determined that lamin A/C protein shows O-glycosylation at G2/M and S phases of the cell cycle. In addition to O-GlcNAcylation and O-GalNAcylation, lamin A/C is found to be contain Gal, Fuc, Man, and Sia sugars at G2/M and S phases for the first time. Having found the glycan units of the lamin A/C protein suggests that glycosylation might have a role in the nuclear organization during the cell cycle.





Similar content being viewed by others
References
Simon, D. N., & Wilson, K. L. (2013). Partners and post-translational modifications of nuclear lamins. Chromosoma, 122, 13–31. https://doi.org/10.1007/s00412-013-0399-8.
Gruenbaum, Y., & Medalia, O. (2015). Lamins: the structure and protein complexes. Current Opinion in Cell Biology, 32, 7–12. https://doi.org/10.1016/j.ceb.2014.09.009.
Lin, F., & Worman, H. J. (1993). Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem., 268(22), 16321–16326. https://doi.org/10.1016/S0021-9258(19)85424-8.
Dechat, T., Adam, S. A., Taimen, P., Shimi, T., & Goldman, R. D. (2010). Nuclear lamins. Cold Spring Harb. Perspect. Biol., 2, 1–22. https://doi.org/10.1101/cshperspect.a000547.
Dittmer, T., & Misteli, T. (2011). The lamin protein family. Genome Biology, 12, 222 https://doi.org/10.1186/gb-2011-12-5-222.
Fawcett, D. W. (1966). On the occurrence of a fibrous lamina on the inner aspect of the nuclear envelope in certain cells of vertebrates. American Journal of Anatomy, 119(1), 129–145. https://doi.org/10.1002/aja.1001190108.
Gruenbaum, Y., Goldman, R. D., Meyuhas, R., Mills, E., Margalit, A., Fridkin, A., Dayani, Y., Prokocimer, M., & Enosh, A. (2003). The Nuclear lamina and its functions in the nucleus. Int. Review of Cytology, 226, 1–62. https://doi.org/10.1016/s0074-7696(03)01001-5.
Dechat, T., Pfleghaar, K., Sengupta, K., Shimi, T., Shumaker, D. K., Solimando, L., & Goldman, R. D. (2008). Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes&Development, 22, 832–853. https://doi.org/10.1101/gad.1652708.
Prokocimer, M., Davidovich, M., Nissim-rafinia, M., Wiesel-motiuk, N., Bar, D. Z., Barkan, R., Meshorer, E., & Gruenbaum, Y. (2009). Nuclear lamins: key regulators of nuclear structure and activities. J. Cell Mol. Med., 13(6), 1059–1085. https://doi.org/10.1111/j.1582-4934.2008.00676.x.
Ho, C. Y., & Lammberding, J. (2012). Lamins at a glance. Journal of Cell Science, 125, 2087–2093. https://doi.org/10.1242/jcs.087288.
Wilson, K. L., & Foisner, R. (2010). Lamin-binding proteins. Cold Spring Harb. Perspect. Biol., 2, a000554 https://doi.org/10.1101/cshperspect.a000554.
Murray-Nerger, L. A., & Cristea, I. M. (2021). Lamin post-translational modifications: emerging toggles of nuclear organization and function. Trends in Biochem. Sci, 46(10), 832–847. https://doi.org/10.1016/j.tibs.2021.05.007.
Gerace, L., & Blobel, G. (1980). The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell, 19(1), 277–287. https://doi.org/10.1016/0092-8674(80)90409-2.
Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., Walter P. (2015) Molecular Biology of The Cell, sixth ed., Garland Science, USA.
Mitra, N., Sinha, S., Ramya, T. N., & Surolia, A. (2006). N-linked oligosaccharides as outfitters for glycoprotein folding, form and function. Trends Biochem. Sci., 31, 156–163. https://doi.org/10.1016/j.tibs.2006.01.003.
Hart, G. W., Kreppel, L. K., Comer, F. I., Arnold, C. S., Snow, D. M., Ye, Z., & Akimoto, Y. (1996). O-GlcNAcylation of key nuclear and cytoskeletal proteins: reciprocity with O-phosphorylation and putative roles in protein multimerization. Glycobiology, 6(7), 711–716. https://doi.org/10.1093/glycob/6.7.711.
Cheng, X., & Hart, G. W. (2001). Alternative O-glycosylation/O-phosphorylation of serine-16 in murine estrogen receptor β post-translational regulation of turnover and transactivation activity. J. Biol. Chem, 276(13), 10570–10575. https://doi.org/10.1074/jbc.M010411200.
Comer, F. I., & Hart, G. W. (2001). Reciprocity between O-GlcNAc and O-phospate on the carboxyl terminal domain of RNA polymerase II. Biochemistry, 40, 7845–7852. https://doi.org/10.1021/bi0027480.
Taylor M. E., Drickamer K. (2003) Introduction to Glycobiology, second ed., Oxford: University Press, UK.
Hardivillé, S., & Hart, G. W. (2014). Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell metabolism, 20(2), 208–213. https://doi.org/10.1016/j.cmet.2014.07.014.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. https://doi.org/10.1038/227680a0.
Burnette, W. N. (1981). “Western Blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem., 112(2), 195–203. https://doi.org/10.1016/0003-2697(81)90281-5.
Shevchenko, A., Tomas, H., Havlis, J., Olsen, J. V., & Mann, M. (2006). In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nature Protocols, 1(6), 2856–2860. https://doi.org/10.1038/nprot.2006.468.
İzzetoğlu, S., Şahar, U., Şener, E., & Deveci, R. (2014). Determination of sialic acids in ımmune system cells (coelomocytes) of sea urchin, Paracentrotus lividus, using capillary LC-ESI-MS/MS. Fish&Shellfish Immunology, 36, 181–186. https://doi.org/10.1016/j.fsi.2013.10.029.
Şener, E., & Deveci, R. (2015). Determining the monosaccharides of the sea urchin (Paracentrotus lividus) coelomocytes via the CapLC-ESI-MS/MS system and the lectin histochemistry. Fish&Shellfish Immunology, 42(1), 34–40. https://doi.org/10.1016/j.fsi.2014.10.020.
Şahar, U., & Deveci, R. (2017). Profiling N-glycans of the egg jelly coat of the sea urchin Paracentrotus lividus by MALDI-TOF mass spectrometry and capillary liquid chromatography electrospray ionization-ion trap tandem mass spectrometry systems. Mol. Reprod. Dev., 84, 401–407. https://doi.org/10.1002/mrd.22794.
Demir, R., Şahar, U., & Deveci, R. (2021). Determination of terminal glycan and total monosaccharide profiles of reelin glycoprotein in SH-SY5Y neuroblastoma cell line by lectin blotting and capillary liquid chromatography electrospray ionization-ion trap tandem mass spectrometry system. BBA-Proteins and Proteomics, 1869, 140559 https://doi.org/10.1016/j.bbapap.2020.140559.
Deveci, R., Şener, E., & İzzetoğlu, S. (2015). Morphological and ultrastructural characterization of sea urchin immune cells. Journal of Morphology, 276, 583–588. https://doi.org/10.1002/jmor.20368.
İzzetoğlu, S., & Karaçalı, S. (2012). The determination of N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc) types of sialic acids in hematopoietic organ of the silkworm. Bombyx mori L. (Lepidoptera: Bombycidae). Kafkas Univ. Vet. Fak. Derg, 18(1), 147–150. https://doi.org/10.9775/kvfd.2011.5257.
Pisano, A., Packer, N. H., Redmond, J. W., Williams, K. L., & Gooley, A. A. (1994). Characterization of O-linked glycosylation motifs in the glycopeptide domain of bovine K-casein. Glycobiology, 4(6), 837–844. https://doi.org/10.1093/glycob/4.6.837.
Atassi M. Z., Appella E. (1995) Methods in protein structure analysis, Springer Science+Business Media, New York.
Anumula, K. R., & Du, P. (1999). Characterization of carbohydrates using highly fluorescent 2-aminobenzoic acid tag following gel electrophoresis of glycoproteins. Analytical Biochemistry, 275, 236–242. https://doi.org/10.1006/abio.1999.4323.
Karve, T. M., & Cheema, A. K. (2011). Small changes huge impact: the role of proteinposttranslational modifications in cellular homeostasis and disease. J. of Amino Acids, 207691, 1–13. https://doi.org/10.4061/2011/207691.
Cejas, R. B., Lorenz, V., Garay, Y. C., & Irazoqui, F. J. (2019). Biosynthesis of O-N-acetylgalactosamine glycans in the human cell nucleus. J. Biol. Chem, 294(9), 2997–3011. https://doi.org/10.1074/jbc.RA118.005524.
Moir, R. D., Spann, T. P., Lopez-Soler, R. I., Yoon, M., Goldman, A. E., Khuon, S., & Goldman, R. D. (2000). The dynamics of the nuclear lamins during the cell cycle—Relationship between structure and function. Journal of Structural Biology, 129, 324–334. https://doi.org/10.1006/jsbi.2000.4251.
Kochin, V., Shimi, T., Torvaldson, E., Adam, S. A., Goldman, A., Pack, C., Melo-Cardenas, J., Imanishi, S. Y., Goldman, R. D., & Eriksson, J. E. (2014). Interphase phosphorylation of lamin A. J. Cell Sci., 127, 2683–2696. https://doi.org/10.1242/jcs.141820.
Wang, S., Huang, X., Sun, D., **n, X., Pan, Q., Peng, S., Liang, Z., Luo, C., Yang, Y., Jiang, H., Huang, M., Chai, W., Ding, J., & Geng, M. (2012). Extensive crosstalk between O-GlcNacylation and phosphorylation regulates Akt signaling. PlosOne, 7(5), e37427 https://doi.org/10.1371/journal.pone.0037427.
Ferraro, A., Eufemi, M., Cervoni, L., Marinetti, R., & Turano, C. (1989). Glycosylated forms of nuclear lamins. FEBS Letters, 257(2), 241–246. https://doi.org/10.1016/0014-5793(89)81543-1.
Wang, Z., Udeshi, N. D., Slawson, C., Compton, P. D., Sakabe, K., Cheung, W. D., Shabanowitz, J., Hunt, D. F., & Hart, G. W. (2010). Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Science Signaling, 3(104), 1–12. https://doi.org/10.1126/scisignal.2000526.
Alfaro, J. F., Gong, C. X., Monroe, M. E., Aldrich, J. T., Clauss, T. R., Purvine, S. O., Wang, Z., Camp, D. G., Shabanowitz, J., Stanley, P., Hart, G. W., Hunt, D. F., Yang, F., & Smith, R. D. (2012). Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets. Proc. Natl. Acad. Sci., 109(19), 7280–7285. https://doi.org/10.1073/pnas.120042510989.
Simon, D. N., Wriston, A., Fan, Q., Shabanowitz, J., Florwick, A., Dharmaraj, T., Peterson, S. B., Gruenbaum, Y., Carlson, C. R., Grønning-Wang, L. M., Hunt, D. F., & Wilson, K. L. (2018). OGT (O-GlcNAc Transferase) selectively modifies multiple residues unique to lamin A. Cells, 7(5), 1–21. https://doi.org/10.3390/cells7050044.
Hart, G. W., Holt, G. D., & Haltiwanger, R. S. (1988). Nuclear and cytoplasmic glycosylation: novel saccharide linkages in unexpected places. Trends in Biochemical Sciences, 13(10), 380–384. https://doi.org/10.1016/0968-0004(88)90179-X.
Haltiwanger, R. S., Kelly, W. G., Roquemore, E. P., Blomberg, M. A., Dong, L. Y. D., Kreppel, L., & Hart, G. W. (1992). Glycosylation of nuclear and cytoplasmic proteins is ubiquitous and dynamic. Biochem. Soc. Trans, 20, 264–269. https://doi.org/10.1042/bst0200264.
Blom, N., Sicheritz-Ponten, T., Gupta, R., Gammeltoft, S., & Brunak, S. (2004). Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics, 4, 1633–1649. https://doi.org/10.1002/pmic.200300771.
Hart, G. W., Housley, M. P., & Slawson, C. (2007). Cycling of O-linked β-N acetylglucosamine on nucleocytoplasmic proteins. Nature, 446(7139), 1017–1022. https://doi.org/10.1038/nature05815.
Varki, A. (2007). Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature, 446(7139), 1023–1029. https://doi.org/10.1038/nature05816.
Hart, G. W. (1997). Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annual Review of Biochem, 66(1), 315–335. https://doi.org/10.1146/annurev.biochem.66.1.315.
Li, B., & Kohler, J. J. (2014). Glycosylation of the nuclear pore. Traffic, 15, 347–361. https://doi.org/10.1111/tra.12150.
West, C. M., Van Der Wel, H., & Gaucher, E. A. (2002). Complex glycosylation of Skp1 in Dictyostelium: implications for the modification of other eukaryotic cytoplasmic and nuclear proteins. Glycobiology, 12(2), 17R–27R. https://doi.org/10.1093/glycob/12.2.17R.
Bond, M. R., & Hanover, J. A. (2015). A little sugar goes a long way: the cell biology of O-GlcNAc. J. Cell Biol, 208, 869–880. https://doi.org/10.1083/jcb.201501101.
Davis, L. I., & Blobel, G. (1987). Nuclear pore complex contains a family of glycoproteins that includes p62: glycosylation through a previously unidentified cellular pathway. Proc. Natl. Acad. Sci, 84(21), 7552–7556. https://doi.org/10.1073/pnas.84.21.7552.
Ferraro, A., Grandi, P., Eufemi, M., Altieri, F., Cervoni, L., & Turano, C. (1991). The presence of N-glycosylated proteins in cell nuclei. Biochemical and Biophysical Research Communications, 178(3), 1365–1370. https://doi.org/10.1016/0006-291X(91)91044-D.
Lefebvre, T., Ferreira, S., Dupont-Wallois, L., Bussiere, T., Dupire, M. J., Delacourte, A., & Caillet-Boudin, M. L. (2003). Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins- a role in nuclear localization. BBA-General Subjects, 1619(2), 167–176. https://doi.org/10.1016/S0304-4165(02)00477-4.
Yang, Z., Halim, A., Narimatsu, Y., Jitendra Joshi, H., Steentoft, C., Schjoldager, K. T., Alder Schulz, M., Sealover, N. R., Kayser, K. J., Bennett, E. P., Levery, S. B., Vakhrushev, S. Y., & Clausen, H. (2014). The GalNAc-type O-glycoproteome of CHO cells characterized by the SimpleCell strategy. Mol. Cell. Proteomics, 13, 3224–3235. https://doi.org/10.1074/mcp.M114.041541.
Uslupehlivan, M., Şener, E., & Deveci, R. (2018). In silico analysis of Pax6 protein glycosylation in vertebrates. Comput. Biol. Chem, 77, 116–122. https://doi.org/10.1016/j.compbiolchem.2018.09.016.
Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E. (2009). Essentials of Glycobiology, second ed., Cold Spring Harbor Laboratory Press, New York.
Taylor M. E., Drickamer K. (2011) Introduction to Glycobiology, third ed., Oxford: University Press, UK.
Clements, L., Manilal, S., Love, D. R., & Morris, G. E. (2000). Direct interaction between emerin and lamin A. Biochemical and Biophysical Research Communications, 267(3), 709–714. https://doi.org/10.1006/bbrc.1999.2023.
Lee, K. K., Haraguchi, T., Lee, R. S., Kou**, T., Hiraoka, Y., & Wilson, K. L. (2001). Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF. J. of Cell Sci., 114(24), 4567–4573. https://doi.org/10.1242/jcs.114.24.4567.
Sakaki, M., Koike, H., Takahashi, N., Sasagawa, N., Tomioka, S., Arahata, K., & Ishiura, S. (2001). Interaction between emerin and nuclear lamins. Journal of Biochemistry, 129(2), 321–327. https://doi.org/10.1093/oxfordjournals.jbchem.a002860.
Brachner, A., Reipert, S., Foisner, R., & Gotzmann, J. (2005). LEM2 is a novel MAN1-related inner nuclear membrane protein associated with A-type lamins. Journal of Cell Science, 118(24), 5797–5810. https://doi.org/10.1242/jcs.02701.
Mansharamani, M., & Wilson, K. L. (2005). Direct binding of nuclear membrane protein MAN1 to emerin in vitro and two modes of binding to barrier-to-autointegration factor. J. Biol. Chem, 280(14), 13863–13870. https://doi.org/10.1074/jbc.M413020200.
Varki, A. (1997). Sialic acids as ligands in recognition phenomena. FASEB J, 11(4), 248–255. https://doi.org/10.1096/fasebj.11.4.9068613.
Varki, A. (2017). Biological roles of glycans. Glycobiology, 27(1), 3–49. https://doi.org/10.1093/glycob/cww086.
Zhou, X., Yang, G., & Guan, F. (2020). Biological functions and analytical strategies of sialic acids in tumor. Cells, 9(2), 273 https://doi.org/10.3390/cells9020273. 1-18.
Higel, F., Seidl, A., Sörgel, F., & Friess, W. (2016). N-Glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur. J. of Pharmaceutics and Biopharmaceutics, 100, 94–100. https://doi.org/10.1016/j.ejpb.2016.01.005.
Strahl-Bolsinger, S., Gentzsch, M., & Tanner, W. (1999). Protein O-mannosylation. Biochimica et Biophysica Acta, 1426, 297–307. https://doi.org/10.1016/S0304-4165(98)00131-7.
Larsen, I. S. B., Narimatsu, Y., Joshi, H. J., Yang, Z., Harrison, O. J., Brasch, J., Shapiro, L., Honig, B., Vakhrushev, S., Clausen, H., & Halim, A. (2017). Mammalian O-mannosylation of cadherins and plexins is independent of protein O-mannosyltransferases 1 and 2. J. Biol. Chem, 292(27), 11586–11598. https://doi.org/10.1074/jbc.M117.794487.
Loibl, M., & Strahl, S. (2013). Protein O-mannosylation: What we have learned from baker’s yeast. Biochimica et Biophysica Acta, 1833, 2438–2446. https://doi.org/10.1016/j.bbamcr.2013.02.008.
Barresi, R., & Campbell, R. (2006). Dystroglycan: from biosynthesis to pathogenesis of human disease. Journal of Science, 119(2), 199–207. https://doi.org/10.1242/jcs.02814.
Praismann, J. L., & Wells, L. (2014). Mammalian O-mannosylation pathway: glycan structures, enzymes, and protein substrates. Biochemistry, 53, 3066–3078. https://doi.org/10.1021/bi500153y.
Vester-Christensen, M. B., Halim, A., Joshi, H. J., Steentoft, C., Bennett, E. P., Levery, S. B., Vakhrushev, S. Y., & Clausen, H. (2013). Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. PNAS, 110(52), 21018–21023. https://doi.org/10.1073/pnas.1313446110.
Hozak, P., Sasseville, A. M. J., Raymond, Y., & Cook, P. R. (1995). Lamin proteins form an internal nucleoskeleton as well as a peripheral lamina in human cells. Journal of Cell Science, 108(2), 635–644. https://doi.org/10.1242/jcs.108.2.635.
Cohen, M., Santarella, R., Wiesel, N., Mattaj, I., & Gruenbaum, Y. (2008). Electron microscopy of lamin and the nuclear lamina in Caenorhabditis elegans. Methods in Cell Biology, 88, 411–429. https://doi.org/10.1016/S0091-679X(08)00421-4.
Acknowledgements
This work was supported by the Ege University Scientific Research Projects Coordination (grant number 17-FEN-031).
Author information
Authors and Affiliations
Contributions
E.Ş.U. performed all the experiments and wrote the manuscript, R.D. performed the TEM preparation studies, U.Ş. performed the CapLC-ESI-MS/MS analysis, S.İ. obtained the TEM micrographs and supervised the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Şener Uslupehlivan, E., Deveci, R., Şahar, U. et al. Glycan analysis of Lamin A/C protein at G2/M and S phases of the cell cycle. Cell Biochem Biophys 80, 689–698 (2022). https://doi.org/10.1007/s12013-022-01102-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-022-01102-3