Abstract
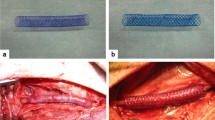
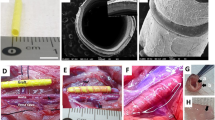
Characterization of biodegradable stent vein graft thickening. Polydioxanone vascular sutures (PDSs) were used in a biodegradable arteriovenous bypass model. Twenty-four rabbits underwent carotid interposition bypass via ipsilateral jugular vein. One half received the stent (PDS group) and the remaining half a simple vein graft (controls). Group subsets received external stent removal or sham-control exploration at 4 and 12 weeks. At 4 and 12 weeks, the PDS group had significantly less medial and intimal thickening than the control group (P < 0.05), and there were fewer proliferating smooth muscle cells and extra cellular matrix formation than the control group at every interval. At 12 weeks, partial stent degradation occurred without deleterious effects. Furthermore proliferating cell nuclear antigen (PCNA), angiotensin type 1 receptor (AT1R), and transforming growth factor beta 1 (TGF-β1) levels were significantly lower than the control group. The external stent inhibited medial and intimal hyperplasia, an effect that remains after the material has completely degraded. This PDS stent is feasible option for vein grafts.





Similar content being viewed by others
References
Wallitt, E. J., Jevon, M., & Hornick, P. I. (2007). Therapeutics of vein graft intimal hyperplasia: 100 years on. Annals of Thoracic Surgery, 84, 317–323.
Violaris, A. G., Newby, A. C., & Angelini, G. D. (1993). Effects of external stenting on wall thickening in arteriovenous bypass grafts. Annals of Thoracic Surgery, 55, 667–671.
Angelini, G. D., Lloyd, C., Bush, R., Johnson, J., & Newby, A. C. (2002). An external, oversized, porous polyester stent reduces vein graft neointima formation, cholesterol concentration, and vascular cell adhesion molecule 1 expression in cholesterol-fed pigs. Journal of Thoracic and Cardiovascular Surgery, 124, 950–956.
George, S. J., Izzat, M. B., Gadsdon, P., Johnson, J. L., Yim, A. P., Wan, S., et al. (2001). Macro-porosity is necessary for the reduction of neointimal and medial thickening by external stenting of porcine saphenous vein bypass grafts. Atherosclerosis, 155, 329–336.
Bunt, T. J. (2001). Vascular graft infections: An update. Cardiovascular Surgery, 9, 225–233.
Jeremy, J. Y., Bulbulia, R., Johnson, J. L., Gadsdon, P., Vijayan, V., Shukla, N., et al. (2004). A bioabsorbable (polyglactin), nonrestrictive, external sheath inhibits porcine saphenous vein graft thickening. Journal of Thoracic and Cardiovascular Surgery, 127, 1766–1772.
Smith, M. J., McClure, M. J., Sell, S. A., Barnes, C. P., Walpoth, B. H., Simpson, D. G., et al. (2008). Suture-reinforced electrospun polydioxanone-elastin small-diameter tubes for use in vascular tissue engineering: A feasibility study. Acta Biomaterialia, 4, 58–66.
Chow, W. N., Simpson, D. G., Bigbee, J. W., & Colello, R. J. (2007). Evaluating neuronal and glial growth on electrospun polarized matrices: Bridging the gap in percussive spinal cord injuries. Neuron Glia Biology, 3, 119–126.
Skodacek, D., Brandau, S., Deutschle, T., Lang, S., & Rotter, N. (2008). Growth factors and scaffold composition influence properties of tissue engineered human septal cartilage implants in a murine model. International Journal of Immunopathology and Pharmacology, 21, 807–816.
Kontio, R., Ruuttila, P., Lindroos, L., Suuronen, R., Salo, A., Lindqvist, C., et al. (2005). Biodegradable polydioxanone and poly(l/d)lactide implants: An experimental study on peri-implant tissue response. International Journal of Oral and Maxillofacial Surgery, 34, 766–776.
Angelini, G. D., Izzat, M. B., Bryan, A. J., & Newby, A. C. (1996). External stenting reduces early medial and neointimal thickening in a pig model of arteriovenous bypass grafting. Journal of Thoracic and Cardiovascular Surgery, 112, 79–84.
Klesius, A. A., Konerding, M. A., Knez, P., Dzemali, O., Schmitz-Rixen, T., Ackermann, H., et al. (2007). External stenting with a new polyester mesh reduces neointimal hyperplasia of vein grafts in a sheep model. International Journal of Artificial Organs, 30, 930–938.
**a, Z., & Triffitt, J. T. (2006). A review on macrophage responses to biomaterials. Biomedical Materials, 1, R1–R9.
Liao, S. W., Lu, X., Putnam, A. J., & Kassab, G. S. (2007). A novel time-varying poly lactic-co glycolic acid external sheath for vein grafts designed under physiological loading. Tissue Engineering, 13, 2855–2862.
Vijayan, V., Shukla, N., Johnson, J. L., Gadsdon, P., Angelini, G. D., Smith, F. C., et al. (2004). Long-term reduction of medial and intimal thickening in porcine saphenous vein grafts with a polyglactin biodegradable external sheath. Journal of Vascular Surgery, 40, 1011–1019.
Barker, S. G., Tilling, L. C., Miller, G. C., Beesley, J. E., Fleetwood, G., Stavri, G. T., et al. (1994). The adventitia and atherogenesis: Removal initiates intimal proliferation in the rabbit which regresses on generation of a ‘neoadventitia’. Atherosclerosis, 105, 131–144.
Martin, J. F., Booth, R. F., & Moncada, S. (1991). Arterial wall hypoxia following thrombosis of the vasa vasorum is an initial lesion in atherosclerosis. European Journal of Clinical Investigation, 21, 355–359.
Jeremy, J. Y., Gadsdon, P., Shukla, N., Vijayan, V., Wyatt, M., Newby, A. C., et al. (2007). On the biology of saphenous vein grafts fitted with external synthetic sheaths and stents. Biomaterials, 28, 895–908.
Khan, R., Agrotis, A., & Bobik, A. (2007). Understanding the role of transforming growth factor-beta1 in intimal thickening after vascular injury. Cardiovascular Research, 74, 223–234.
Schmidt, A., Lorkowski, S., Seidler, D., Breithardt, G., & Buddecke, E. (2006). TGF-beta1 generates a specific multicomponent extracellular matrix in human coronary SMC. European Journal of Clinical Investigation, 36, 473–482.
Yao, E. H., Fukuda, N., Ueno, T., Matsuda, H., Nagase, H., Matsumoto, Y., et al. (2009). A pyrrole-imidazole polyamide targeting transforming growth factor-beta1 inhibits restenosis and preserves endothelialization in the injured artery. Cardiovascular Research, 81, 797–804.
Stracke, S., Konner, K., Kostlin, I., Friedl, R., Jehle, P. M., Hombach, V., et al. (2002). Increased expression of TGF-beta1 and IGF-I in inflammatory stenotic lesions of hemodialysis fistulas. Kidney International, 61, 1011–1019.
Joki, N., Kaname, S., Hirakata, M., Hori, Y., Yamaguchi, T., Fujita, T., et al. (2000). Tyrosine-kinase dependent TGF-beta and extracellular matrix expression by mechanical stretch in vascular smooth muscle cells. Hypertension Research, 23, 91–99.
Barker, T. A., Massett, M. P., Korshunov, V. A., Mohan, A. M., Kennedy, A. J., & Berk, B. C. (2006). Angiotensin II type 2 receptor expression after vascular injury: Differing effects of angiotensin-converting enzyme inhibition and angiotensin receptor blockade. Hypertension, 48, 942–949.
Dubey, R. K., Jackson, E. K., & Luscher, T. F. (1995). Nitric oxide inhibits angiotensin II-induced migration of rat aortic smooth muscle cell. Role of cyclic-nucleotides and angiotensin1 receptors. Journal of Clinical Investigation, 96, 141–149.
Nishimoto, M., Takai, S., Sawada, Y., Yuda, A., Kondo, K., Yamada, M., et al. (2001). Chymase-dependent angiotensin II formation in the saphenous vein versus the internal thoracic artery. Journal of Thoracic and Cardiovascular Surgery, 121, 729–734.
Acknowledgments
This study was supported by China Postdoctoral Science Foundation (20070410732), and Jiangsu Nature Science Foundation (BK2007543).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
You, Q., Duan, L., Wang, F. et al. Characterization of the Inhibition of Vein Graft Intimal Hyperplasia by a Biodegradable Vascular Stent. Cell Biochem Biophys 59, 99–107 (2011). https://doi.org/10.1007/s12013-010-9118-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-010-9118-8