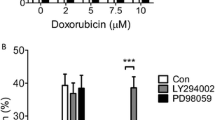
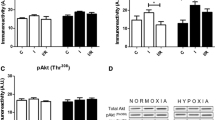
Abstract
Intermittent hypoxic preconditioning (IHP) is a well-established cardioprotective intervention in models of ischemia/reperfusion injury. Nevertheless, the significance of IHP in different cardiac pathologies remains elusive. In order to investigate the role of IHP and its effects on calcium-dependent signalization in HF, we employed a model of cardiomyopathy induced by doxorubicin (Dox), a widely used drug from the class of cardiotoxic antineoplastics, which was i.p. injected to Wistar rats (4 applications of 4 mg/kg/week). IHP-treated group was exposed to IHP for 2 weeks prior to Dox administration. IHP ameliorated Dox-induced reduction in cardiac output. Western blot analysis revealed increased expression of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2a) while the expression of hypoxia inducible factor (HIF)-1-α, which is a crucial regulator of hypoxia-inducible genes, was not changed. Animals administered with Dox had further decreased expression of TRPV1 and TRPV4 (transient receptor potential, vanilloid subtype) ion channels along with suppressed Ca2+/calmodulin-dependent protein kinase II (CaMKII) activation. In summary, IHP-mediated improvement in cardiac output in the model of Dox-induced cardiomyopathy is likely a result of increased SERCA2a expression which could implicate IHP as a potential protective intervention in Dox cardiomyopathy, however, further analysis of observed effects is still required.








Similar content being viewed by others
Data Availability
The raw data supporting the conclusions of this article will be made available by the authors on reasonable request.
References
Chung, W. B., & Youn, H. J. (2016). Pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity. The Korean Journal of Internal Medicine, 31(4), 625–633. https://doi.org/10.3904/kjim.2016.017
Volkova, M., & Russell, R., 3rd. (2011). Anthracycline cardiotoxicity: Prevalence, pathogenesis and treatment. Current Cardiology Reviews, 7(4), 214–220. https://doi.org/10.2174/157340311799960645
Rivera, E. (2003). Liposomal anthracyclines in metastatic breast cancer: Clinical update. The oncologist, 8(Suppl 2), 3–9. https://doi.org/10.1634/theoncologist.8-suppl_2-3
Martins-Teixeira, M. B., & Carvalho, I. (2020). Antitumour anthracyclines: progress and perspectives. ChemMedChem, 15(11), 933–948. https://doi.org/10.1002/cmdc.202000131
Shevchuk, O. O., Posokhova, E. A., Sakhno, L. A., & Nikolaev, V. G. (2012). Theoretical ground for adsorptive therapy of anthracyclines cardiotoxicity. Experimental Oncology, 34(4), 314–322.
Nebigil, C. G., & Désaubry, L. (2018). Updates in anthracycline-mediated cardiotoxicity. Frontiers in Pharmacology, 9, 1262. https://doi.org/10.3389/fphar.2018.01262
Sag, C. M., Köhler, A. C., Anderson, M. E., Backs, J., & Maier, L. S. (2011). CaMKII-dependent SR Ca leak contributes to doxorubicin-induced impaired Ca handling in isolated cardiac myocytes. Journal of Molecular and Cellular Cardiology, 51(5), 749–759. https://doi.org/10.1016/j.yjmcc.2011.07.016
Llach, A., Mazevet, M., Mateo, P., Villejouvert, O., Ridoux, A., Rucker-Martin, C., Ribeiro, M., Fischmeister, R., Crozatier, B., Benitah, J. P., Morel, E., & Gómez, A. M. (2019). Progression of excitation-contraction coupling defects in doxorubicin cardiotoxicity. Journal of Molecular and Cellular Cardiology, 126, 129–139. https://doi.org/10.1016/j.yjmcc.2018.11.019
Pecoraro, M., Rodríguez-Sinovas, A., Marzocco, S., Ciccarelli, M., Iaccarino, G., Pinto, A., & Popolo, A. (2017). Cardiotoxic effects of short-term doxorubicin administration: involvement of connexin 43 in calcium impairment. International Journal of Molecular Sciences, 18(10), 2121. https://doi.org/10.3390/ijms18102121
Fernandez-Chas, M., Curtis, M. J., & Niederer, S. A. (2018). Mechanism of doxorubicin cardiotoxicity evaluated by integrating multiple molecular effects into a biophysical model. British Journal of Pharmacology, 175(5), 763–781. https://doi.org/10.1111/bph.14104
Zhu, W., Reuter, S., & Field, L. J. (2019). Targeted expression of cyclin D2 ameliorates late stage anthracycline cardiotoxicity. Cardiovascular Research, 115(5), 960–965. https://doi.org/10.1093/cvr/cvy273
Kraft, J., Grille, W., Appelt, M., Hossfeld, D. K., Eichelbaum, M., Koslowski, B., Quabeck, K., Kuse, R., Büchner, T., & Hiddemann, W. (1990). Effects of verapamil on anthracycline-induced cardiomyopathy: Preliminary results of a prospective multicenter trial. Haematology and Blood Transfusion, 33, 566–570. https://doi.org/10.1007/978-3-642-74643-7_103
Milei, J., Marantz, A., Alé, J., Vazquez, A., & Buceta, J. E. (1987). Prevention of adriamycin-induced cardiotoxicity by prenylamine: A pilot double blind study. Cancer Drug Delivery, 4(2), 129–136. https://doi.org/10.1089/cdd.1987.4.129
Lu, M. J., Chen, Y. S., Huang, H. S., & Ma, M. C. (2014). Hypoxic preconditioning protects rat hearts against ischemia-reperfusion injury via the arachidonate12-lipoxygenase/transient receptor potential vanilloid 1 pathway. Basic Research in Cardiology, 109(4), 414. https://doi.org/10.1007/s00395-014-0414-0
Hu, K., Deng, W., Yang, J., Wei, Y., Wen, C., Li, X., Chen, Q., Ke, D., & Li, G. (2020). Intermittent hypoxia reduces infarct size in rats with acute myocardial infarction: A systematic review and meta-analysis. BMC Cardiovascular Disorders, 20(1), 422. https://doi.org/10.1186/s12872-020-01702-y
Jarrard, C. P., Nagel, M. J., Stray-Gundersen, S., Tanaka, H., & Lalande, S. (2021). Hypoxic preconditioning attenuates ischemia-reperfusion injury in young healthy adults. Journal of Applied Physiology (Bethesda, Md: 1985), 130(3), 846–852. https://doi.org/10.1152/japplphysiol.00772.2020
Baumgarten, K. M., Gerstenblith, G., & Weiss, R. G. (1999). High extracellular K+ during hypoxic preconditioning episodes attenuates the post-ischemic contractile and ionic benefits of preconditioning. Journal of Molecular and Cellular Cardiology, 31(1), 203–213. https://doi.org/10.1006/jmcc.1998.0860
Liu, X., Xu, F., Fu, Y., Liu, F., Sun, S., & Wu, X. (2006). Calreticulin induces delayed cardioprotection through mitogen-activated protein kinases. Proteomics, 6(13), 3792–3800. https://doi.org/10.1002/pmic.200500906
Wu, X., Liu, X., Zhu, X., & Tang, C. (2007). Hypoxic preconditioning induces delayed cardioprotection through p38 MAPK-mediated calreticulin upregulation. Shock (Augusta, Ga), 27(5), 572–577. https://doi.org/10.1097/01.shk.0000246901.58068.a8
Cai, Z., Manalo, D. J., Wei, G., Rodriguez, E. R., Fox-Talbot, K., Lu, H., Zweier, J. L., & Semenza, G. L. (2003). Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation, 108(1), 79–85. https://doi.org/10.1161/01.CIR.0000078635.89229.8A
Tanaka, T., Yamaguchi, J., Shoji, K., & Nangaku, M. (2012). Anthracycline inhibits recruitment of hypoxia-inducible transcription factors and suppresses tumor cell migration and cardiac angiogenic response in the host. The Journal of Biological Chemistry, 287(42), 34866–34882. https://doi.org/10.1074/jbc.M112.374587
Graziani, S., Scorrano, L., & Pontarin, G. (2022). Transient exposure of endothelial cells to doxorubicin leads to long-lasting vascular endothelial growth factor receptor 2 downregulation. Cells, 11(2), 210. https://doi.org/10.3390/cells11020210
Chiusa, M., Hool, S. L., Truetsch, P., Djafarzadeh, S., Jakob, S. M., Seifriz, F., Scherer, S. J., Suter, T. M., Zup**er, C., & Zbinden, S. (2012). Cancer therapy modulates VEGF signaling and viability in adult rat cardiac microvascular endothelial cells and cardiomyocytes. Journal of Molecular and Cellular Cardiology, 52(5), 1164–1175. https://doi.org/10.1016/j.yjmcc.2012.01.022
Huang, Y., Hickey, R. P., Yeh, J. L., Liu, D., Dadak, A., Young, L. H., Johnson, R. S., & Giordano, F. J. (2004). Cardiac myocyte-specific HIF-1alpha deletion alters vascularization, energy availability, calcium flux, and contractility in the normoxic heart. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 18(10), 1138–1140. https://doi.org/10.1096/fj.04-1510fje
Silter, M., Kögler, H., Zieseniss, A., Wilting, J., Schäfer, K., Toischer, K., Rokita, A. G., Breves, G., Maier, L. S., & Katschinski, D. M. (2010). Impaired Ca(2+)-handling in HIF-1alpha(+/-) mice as a consequence of pressure overload. Pflugers Archiv : European Journal of Physiology, 459(4), 569–577. https://doi.org/10.1007/s00424-009-0748-x
Rath, G., Saliez, J., Behets, G., Romero-Perez, M., Leon-Gomez, E., Bouzin, C., Vriens, J., Nilius, B., Feron, O., & Dessy, C. (2012). Vascular hypoxic preconditioning relies on TRPV4-dependent calcium influx and proper intercellular gap junctions communication. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(9), 2241–2249. https://doi.org/10.1161/ATVBAHA.112.252783
Hof, T., Chaigne, S., Récalde, A., Sallé, L., Brette, F., & Guinamard, R. (2019). Transient receptor potential channels in cardiac health and disease. Nature Reviews. Cardiology, 16(6), 344–360. https://doi.org/10.1038/s41569-018-0145-2
Kang, Y., Wang, W., Zhao, H., Qiao, Z., Shen, X., & He, B. (2017). Assessment of Subclinical Doxorubicin-induced Cardiotoxicity in a Rat Model by Speckle-Tracking Imaging. Arquivos brasileiros de cardiologia. https://doi.org/10.5935/abc.20170097
**, L., Tekin, D., Gursoy, E., Salloum, F., Levasseur, J. E., & Kukreja, R. C. (2002). Evidence that NOS2 acts as a trigger and mediator of late preconditioning induced by acute systemic hypoxia. American Journal of Physiology. Heart and Circulatory Physiology, 283(1), 5–12. https://doi.org/10.1152/ajpheart.00920.2001
Szobi, A., Gonçalvesová, E., Varga, Z. V., Leszek, P., Kuśmierczyk, M., Hulman, M., Kyselovič, J., Ferdinandy, P., & Adameová, A. (2017). Analysis of necroptotic proteins in failing human hearts. Journal of Translational Medicine, 15(1), 86. https://doi.org/10.1186/s12967-017-1189-5
Moritz, C. P. (2017). Tubulin or not tubulin: heading toward total protein staining as loading control in western blots. Proteomics. https://doi.org/10.1002/pmic.201600189
Curtis, M. J., Alexander, S., Cirino, G., Docherty, J. R., George, C. H., Giembycz, M. A., Hoyer, D., Insel, P. A., Izzo, A. A., Ji, Y., MacEwan, D. J., Sobey, C. G., Stanford, S. C., Teixeira, M. M., Wonnacott, S., & Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. https://doi.org/10.1111/bph.14153
Rathod, N., Bak, J. J., Primeau, J. O., Fisher, M. E., Espinoza-Fonseca, L. M., Lemieux, M. J., & Young, H. S. (2021). Nothing regular about the regulins: distinct functional properties of SERCA transmembrane peptide regulatory subunits. International Journal of Molecular Sciences, 22(16), 8891. https://doi.org/10.3390/ijms22168891
Chang, H. H., Hsu, S. P., & Chien, C. T. (2019). Intrarenal transplantation of hypoxic preconditioned mesenchymal stem cells improves glomerulonephritis through anti-oxidation, anti-ER stress, anti-inflammation, anti-apoptosis, and anti-autophagy. Antioxidants (Basel, Switzerland), 9(1), 2. https://doi.org/10.3390/antiox9010002
Chang, J. C., Lien, C. F., Lee, W. S., Chang, H. R., Hsu, Y. C., Luo, Y. P., Jeng, J. R., Hsieh, J. C., & Yang, K. T. (2019). Intermittent hypoxia prevents myocardial mitochondrial Ca2+ overload and cell death during ischemia/reperfusion: The role of reactive oxygen species. Cells, 8(6), 564. https://doi.org/10.3390/cells8060564
Zhong, N., Zhang, Y., Zhu, H. F., & Zhou, Z. N. (2000). Intermittent hypoxia exposure prevents mtDNA deletion and mitochondrial structure damage produced by ischemia/reperfusion injury. Sheng li xue bao: Acta physiologica Sinica, 52(5), 375–380.
Wang, Z., & Si, L. Y. (2013). Hypoxia-inducible factor-1α and vascular endothelial growth factor in the cardioprotective effects of intermittent hypoxia in rats. Upsala Journal of Medical Sciences, 118(2), 65–74. https://doi.org/10.3109/03009734.2013.766914
Semenza, G. L. (2014). Hypoxia-inducible factor 1 and cardiovascular disease. Annual Review of Physiology, 76, 39–56. https://doi.org/10.1146/annurev-physiol-021113-170322
Li, J., Li, C., Yuan, W., Wu, J., Li, J., Li, Z., & Zhao, Y. (2021). Targeted temperature management suppresses hypoxia-inducible factor-1α and vascular endothelial growth factor expression in a pig model of cardiac arrest. Neurocritical caRe, 35(2), 379–388. https://doi.org/10.1007/s12028-020-01166-0
**e, H. C., Li, J. G., & He, J. P. (2017). Differential responsiveness in VEGF receptor subtypes to hypoxic stress in various tissues of plateau animals. Physiological Research, 66(2), 357–362. https://doi.org/10.33549/physiolres.933408
Lin, J. S., Chen, Y. S., Chiang, H. S., & Ma, M. C. (2008). Hypoxic preconditioning protects rat hearts against ischaemia-reperfusion injury: Role of erythropoietin on progenitor cell mobilization. The Journal of Physiology, 586(23), 5757–5769. https://doi.org/10.1113/jphysiol.2008.160887
Simůnek, T., Sterba, M., Holecková, M., Kaplanová, J., Klimtová, I., Adamcová, M., Gersl, V., & Hrdina, R. (2005). Myocardial content of selected elements in experimental anthracycline-induced cardiomyopathy in rabbits. Biometals : An International Journal on the Role of Metal Ions in Biology, Biochemistry, and Medicine, 18(2), 163–169. https://doi.org/10.1007/s10534-004-4491-7
Babick, A. P., Cantor, E. J., Babick, J. T., Takeda, N., Dhalla, N. S., & Netticadan, T. (2004). Cardiac contractile dysfunction in J2N-k cardiomyopathic hamsters is associated with impaired SR function and regulation. American Journal of Physiology. Cell Physiology, 287(5), C1202–C1208. https://doi.org/10.1152/ajpcell.00155.2004
Ji, Y., Lalli, M. J., Babu, G. J., Xu, Y., Kirkpatrick, D. L., Liu, L. H., Chiamvimonvat, N., Walsh, R. A., Shull, G. E., & Periasamy, M. (2000). Disruption of a single copy of the SERCA2 gene results in altered Ca2+ homeostasis and cardiomyocyte function. The Journal of Biological Chemistry, 275(48), 38073–38080. https://doi.org/10.1074/jbc.M004804200
Ezzitouny, M., Roselló-Lletí, E., Portolés, M., Sánchez-Lázaro, I., Arnau-Vives, M. Á., Tarazón, E., Gil-Cayuela, C., Lozano-Edo, S., López-Vilella, R., Almenar-Bonet, L., & Martínez-Dolz, L. (2021). Value of SERCA2a as a biomarker for the identification of patients with heart failure requiring circulatory support. Journal of Personalized Medicine, 11(11), 1122. https://doi.org/10.3390/jpm11111122
Samuel, T. J., Rosenberry, R. P., Lee, S., & Pan, Z. (2018). Correcting calcium dysregulation in chronic heart failure using SERCA2a gene therapy. International Journal of Molecular Sciences, 19(4), 1086. https://doi.org/10.3390/ijms19041086
Lenčová-Popelová, O., Jirkovský, E., Mazurová, Y., Lenčo, J., Adamcová, M., Šimůnek, T., Geršl, V., & Štěrba, M. (2014). Molecular remodeling of left and right ventricular myocardium in chronic anthracycline cardiotoxicity and post-treatment follow up. PLoS One, 9(5), e96055. https://doi.org/10.1371/journal.pone.0096055
Matsuda, N., Morgan, K. G., & Sellke, F. W. (1999). Preconditioning improves cardioplegia-related coronary microvascular smooth muscle hypercontractility: Role of KATP channels. The Journal of Thoracic and Cardiovascular Surgery, 118(3), 438–445. https://doi.org/10.1016/S0022-5223(99)70180-7
Westberg, J. A., Serlachius, M., Lankila, P., & Andersson, L. C. (2007). Hypoxic preconditioning induces elevated expression of stanniocalcin-1 in the heart. American Journal of Physiology. Heart and Circulatory Physiology, 293(3), H1766–H1771. https://doi.org/10.1152/ajpheart.00017.2007
Chen, R. C., Sun, G. B., Ye, J. X., Wang, J., Zhang, M. D., & Sun, X. B. (2017). Salvianolic acid B attenuates doxorubicin-induced ER stress by inhibiting TRPC3 and TRPC6 mediated Ca2+ overload in rat cardiomyocytes. Toxicology Letters, 276, 21–30. https://doi.org/10.1016/j.toxlet.2017.04.010
Takada, A., Miki, T., Kuno, A., Kouzu, H., Sunaga, D., Itoh, T., Tanno, M., Yano, T., Sato, T., Ishikawa, S., & Miura, T. (2012). Role of ER stress in ventricular contractile dysfunction in type 2 diabetes. PLoS One, 7(6), e39893. https://doi.org/10.1371/journal.pone.0039893
Wu, X. D., Zhang, Z. Y., Sun, S., Li, Y. Z., Wang, X. R., Zhu, X. Q., Li, W. H., & Liu, X. H. (2013). Hypoxic preconditioning protects microvascular endothelial cells against hypoxia/reoxygenation injury by attenuating endoplasmic reticulum stress. Apoptosis : An International Journal on Programmed Cell Death, 18(1), 85–98. https://doi.org/10.1007/s10495-012-0766-6
Wang, J., Wu, Y., Yuan, F., Liu, Y., Wang, X., Cao, F., Zhang, Y., & Wang, S. (2016). Chronic intermittent hypobaric hypoxia attenuates radiation induced heart damage in rats. Life Sciences, 160, 57–63. https://doi.org/10.1016/j.lfs.2016.07.002
Hamstra, S. I., Whitley, K. C., Baranowski, R. W., Kurgan, N., Braun, J. L., Messner, H. N., & Fajardo, V. A. (2020). The role of phospholamban and GSK3 in regulating rodent cardiac SERCA function. American Journal of Physiology. Cell Physiology, 319(4), C694–C699. https://doi.org/10.1152/ajpcell.00318.2020
Tscheschner, H., Meinhardt, E., Schlegel, P., Jungmann, A., Lehmann, L. H., Müller, O. J., Most, P., Katus, H. A., & Raake, P. W. (2019). CaMKII activation participates in doxorubicin cardiotoxicity and is attenuated by moderate GRP78 overexpression. PLoS One, 14(4), e0215992. https://doi.org/10.1371/journal.pone.0215992
Anderson, M. E., Brown, J. H., & Bers, D. M. (2011). CaMKII in myocardial hypertrophy and heart failure. Journal of Molecular and Cellular Cardiology, 51(4), 468–473. https://doi.org/10.1016/j.yjmcc.2011.01.012
Zhang, T., Zhang, Y., Cui, M., **, L., Wang, Y., Lv, F., Liu, Y., Zheng, W., Shang, H., Zhang, J., Zhang, M., Wu, H., Guo, J., Zhang, X., Hu, X., Cao, C. M., & **ao, R. P. (2016). CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nature Medicine, 22(2), 175–182. https://doi.org/10.1038/nm.4017
Rajtik, T., Carnicka, S., Szobi, A., Giricz, Z., O-Uchi, J., VHassova, Svec, P., Ferdinandy, P., Ravingerova, T., & Adameova, A. (2016). Oxidative activation of CaMKIIδ in acute myocardial ischemia/reperfusion injury: A role of angiotensin AT1 receptor-NOX2 signaling axis. European Journal of Pharmacology, 771, 114–122. https://doi.org/10.1016/j.ejphar.2015.12.024
Szobi, A., Rajtik, T., Carnicka, S., Ravingerova, T., & Adameova, A. (2014). Mitigation of postischemic cardiac contractile dysfunction by CaMKII inhibition: Effects on programmed necrotic and apoptotic cell death. Molecular and Cellular Biochemistry, 388(1–2), 269–276. https://doi.org/10.1007/s11010-013-1918-x
Koncsos, G., Varga, Z. V., Baranyai, T., Boengler, K., Rohrbach, S., Li, L., Schlüter, K. D., Schreckenberg, R., Radovits, T., Oláh, A., Mátyás, C., Lux, Á., Al-Khrasani, M., Komlódi, T., Bukosza, N., Máthé, D., Deres, L., Barteková, M., Rajtík, T., Adameová, A., … Ferdinandy, P. (2016). Diastolic dysfunction in prediabetic male rats: Role of mitochondrial oxidative stress. American journal of physiology. Heart and circulatory physiology, 311(4), H927–H943. https://doi.org/10.1152/ajpheart.00049.2016.
Woolums, B. M., McCray, B. A., Sung, H., Tabuchi, M., Sullivan, J. M., Ruppell, K. T., Yang, Y., Mamah, C., Aisenberg, W. H., Saavedra-Rivera, P. C., Larin, B. S., Lau, A. R., Robinson, D. N., **ang, Y., Wu, M. N., Sumner, C. J., & Lloyd, T. E. (2020). TRPV4 disrupts mitochondrial transport and causes axonal degeneration via a CaMKII-dependent elevation of intracellular Ca2. Nature Communications, 11(1), 2679. https://doi.org/10.1038/s41467-020-16411-5
Zhang, S., Lu, K., Yang, S., Wu, Y., Liao, J., Lu, Y., Wu, Q., Zhao, N., Dong, Q., Chen, L., & Du, Y. (2021). Activation of transient receptor potential vanilloid 4 exacerbates myocardial ischemia-reperfusion injury via JNK-CaMKII phosphorylation pathway in isolated mice hearts. Cell Calcium, 100, 102483. https://doi.org/10.1016/j.ceca.2021.102483
Adapala, R. K., Kanugula, A. K., Paruchuri, S., Chilian, W. M., & Thodeti, C. K. (2020). TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation. Basic Research in Cardiology, 115(2), 14. https://doi.org/10.1007/s00395-020-0775-5
Horton, J. S., Buckley, C. L., & Stokes, A. J. (2013). Successful TRPV1 antagonist treatment for cardiac hypertrophy and heart failure in mice. Channels (Austin, Tex.), 7(1), 17–22. https://doi.org/10.4161/chan.23006
Iwata, Y., Wakabayashi, S., Ito, S., & Kitakaze, M. (2020). Production of TRPV2-targeting functional antibody ameliorating dilated cardiomyopathy and muscular dystrophy in animal models. Laboratory Investigation; A Journal of Technical Methods and Pathology, 100(2), 324–337. https://doi.org/10.1038/s41374-019-0363-1
Zhang, Q., Qi, H., Cao, Y., Shi, P., Song, C., Ba, L., Chen, Y., Gao, J., Li, S., Li, B., & Sun, H. (2018). Activation of transient receptor potential vanilloid 3 channel (TRPV3) aggravated pathological cardiac hypertrophy via calcineurin/NFATc3 pathway in rats. Journal of Cellular and Molecular Medicine, 22(12), 6055–6067. https://doi.org/10.1111/jcmm.13880
Acknowledgements
This research was supported by the Faculty of Pharmacy, Comenius University, Bratislava [FaF UK/13/2021]; Scientific Grant Agency of The Ministry of Education of Slovak Republic [VEGA 1/0775/21 and 2/0151/17]; and the Slovak Research And Development Agency [APVV-15-0607].
Author information
Authors and Affiliations
Contributions
Conceptualization, TR, VF, AS and AD-A; writing—original draft preparation, PG, LB, TR; writing—review and editing, PG; TR, LB, VF, CH, DK and AD-A; visualization, TR, LB, and PG; supervision, TR, VF, CH; project administration, TR, VF, DK and AD-A; funding acquisition, TR, VF and AD-A, performing experiments, TR, PG, LB, and DK. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest that are relevant to the content of this manuscript.
Ethical Approval
All procedures conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85‐23, revised 1996) and have been authorized by the ethical commission of the Institute of Experimental Pharmacology and Toxicology of the Slovak Academy of Sciences and State Veterinary and Food Administration of the Slovak Republic (No. 1972/17-221).
Consent to Participate
Not applicable.
Consent for Publication
All the authors are ready to publish the manuscript in ‘Cardiovascular Toxicology’ as per rule and regulations of the journal.
Additional information
Handling Editor: Dakshesh Patel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Galis, P., Bartosova, L., Farkasova, V. et al. Intermittent Hypoxic Preconditioning Plays a Cardioprotective Role in Doxorubicin-Induced Cardiomyopathy. Cardiovasc Toxicol 23, 185–197 (2023). https://doi.org/10.1007/s12012-023-09793-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-023-09793-7