Abstract
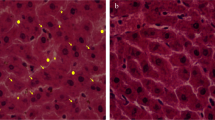
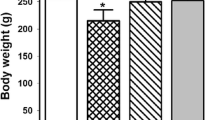
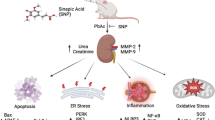
Kidneys are primarily sensitive to lead (Pb) poisoning due to their cardinal role in lead excretion. Then, we studied the effect of glutamine (Gln) on lead nephrotoxicity in rats by assessing the histopathological and biochemical parameters (the renal NF-kβ expression, metabolic profile, oxidative stress, inflammatory markers, methylglyoxal (MGO), and glyoxalase-I activity). Forty rats were allotted into four groups (ten rats in each): normal (N), Gln-treated N, Pb intoxication (Pbi), and Gln-treated Pbi. The treated groups took 0.1% Gln in drinking water for 1 month. To motivate lead poisoning, rats gained 50 mg/l lead acetate in drinking water for 1 month. Oxidative stress indices (total glutathione, its reduced and oxidized forms, their ratios, advanced protein oxidation products, malondialdehyde, and ferric ion reducing power) and inflammatory markers (renal nuclear factor-kβ expression, interleukin 1β level, and myeloperoxidase activity) were measured. Furthermore, metabolic profile (fasting blood sugar, insulin, insulin resistance, lipid profile, and atherogenic index) and renal dysfunction parameters were determined. Pb-induced renal histopathological alterations were investigated by a pathologist. In the kidney of Pbi rats, the glomerulus was damaged. Gln prevented kidney damage and reduced kidney dysfunction parameters. In addition, Gln decreased oxidative stress and inflammation in sera and kidney homogenates. In addition, it improved insulin resistance, dyslipidemia, and carbonyl stress (p < 0.001). Gln guarded the kidneys versus lead intoxication by improving insulin resistance and dyslipidemia, elevating antioxidant markers, and diminishing inflammation and carbonyl stress.

Similar content being viewed by others
References
Albarakati AJ, Baty RS, Aljoudi AM, Habotta OA, Elmahallawy EK, Kassab RB et al (2020) Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, anti-inflammatory, antiapoptotic, and Nrf2/HO1 signaling pathways. Mol Biol Rep 47(4):2591–2603
Alhusaini A, Fadda L, Hasan IH, Zakaria E, Alenazi AM, Mahmoud AM (2019) Curcumin ameliorates lead-induced hepatotoxicity by suppressing oxidative stress and inflammation, and modulating Akt/GSK-3 signaling pathway. Biomolecules 9(11):702–718
Fioresi M, Simoes MR, Furieri LB et al (2014) Chronic lead exposure increases blood pressure and myocardial contractility in rats. PLoS One 9(5):96900
Soussi A, Gargouri M, Akrouti A, El Feki A (2018) Antioxidant and nephroprotective effect of Juglans regia vegetable oil against lead-induced nephrotoxicity in rats and its characterization by GCMS. EXCLI J 17:492–504
Apaydın FG, Baş H, Kalender S, Kalender Y (2016) Subacute effects of low dose lead nitrate and mercury chloride exposure on kidney of rats. Environ Toxicol Pharmacol 41:219–224
Missoun F, Slimani M, Aoues A (2010) Toxic effect of lead on kidney function in rat Wistar. Afr J Biochem Res 4(2):21–27
Ritz E, Mann J, Stoeppler M (1988) Lead and the kidney. Adv Nephrol Necker Hosp 17:241–274
Piuri G, Basello K, Rossi G, Soldavini CM, Duiella S, Privitera G et al (2020) Methylglyoxal, glycated albumin, PAF, and TNF-α: possible inflammatory and metabolic biomarkers for management of gestational diabetes. Nutrients 12(2):479
Mahdavifard S, Dehghani R, Jeddi F, Najafzadeh N (2021) Thiamine reduced metabolic syndrome symptoms in rats via down-regulation of hepatic nuclear factor-kβ and induction activity of glyoxalase-I. Iran J Basic Med Sci 24:46–53
Sun B, Karin M (2008) NF-kB signaling, liver disease and hepatoprotective agents. Oncogene 27:6228–6244
Maessen DE, Stehouwer CD, Schalkwijk CG (2015) The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin Sci 128:839–861
Rabbani N, Xue M, Weickert MO, Thornalley PJ (2021) Reversal of insulin resistance in overweight and obese subjects by trans-resveratrol and hesperetin combination-link to dysglycemia, blood pressure, dyslipidemia, and low-grade inflammation. Nutrients 11(13):2374
Mey JT, H.J. (2018) Dicarbonyl stress and glyoxalase-1 in skeletal muscle: implications for insulin resistance and type 2 diabetes. Front Cardiovasc Med 5:117
Tezuka Y, Nakaya I, Nakayama K, Nakayama M, Yahata M, Soma J (2018) Methylglyoxal as a prognostic factor in patients with chronic kidney disease. Nephrology (Carlton) 24(9):943–950
Pácal L, Chalásová K, Pleskačová A, Řehořová J, Tomandl J, Kaňková K (2018) Deleterious effect of advanced CKD on glyoxalase system activity not limited to diabetes aetiology. Int J Mol Sci 18(19):1517
Kawanami D, Matoba K, Utsunomiya K (2016) Dyslipidemia in diabetic nephropathy. Ren Replace Ther 2(1):1–9
Hasanuzzaman M, Nahar K, Rahman A, Mahmud JA, Alharby HF, Fujita M (2018) Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J Plant Interact 13(1):203–212
Mahdavifard S, S.N. (2022) Glutamine is a superior protector against lead-induced hepatotoxicity in rats via antioxidant, anti-inflammatory, and chelating properties. Biol Trace Elem Res 200(11):4726–4732
Newairy AS, Abdou HM (2009) Protective role of flax lignans against lead acetate induced oxidative damage and hyperlipidemia in rats. Food Chem Toxicol 47(4):813–818
Yun S et al (2019) Effects of lead exposure on brain glucose metabolism and insulin signaling pathway in the hippocampus of rats. Toxicol Lett 310:23–30
Flora SJP, V. (2010) Chelation in metal intoxication. Int J Environ Res Public Health 7:2745–2788
Mohamed RS, Fouda K, Akl EM (2020) Hepatorenal protective effect of flaxseed protein isolate incorporated in lemon juice against lead toxicity in rats. Toxicol Rep 7:30–35
Jalali M (2020) Effect of iron-amino acid chelates on antioxidant capacity and nutritional value of soybean. Plant Prod 43(4):477–486
Kim J, Lee Y, Yang M (2014) Environmental exposure to lead (Pb) and variations in its susceptibility. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 32:159–185
Mima A, Matsubara T, Arai H, Abe H, Nagai K, Kanamori H et al (2006) Angiotensin II-dependent Src and Smad1 signaling pathway is crucial for the development of diabetic nephropathy. Lab Investig 86:927–939
Song YS et al (2016) Comparison of the usefulness of the updated homeostasis model assessment (HOMA2) with the original HOMA1 in the prediction of type 2 diabetes mellitus in Koreans. Diabetes Metab J 40(4):318–325
Taylor EL, Armstrong KR, Perrett D, Hattersley AT, Winyard PG (2015) Optimisation of an advanced oxidation protein products assay: its application to studies of oxidative stress in diabetes mellitus. Oxidative Med Cell Longev 2015:496271
Ahotupa M, Marniemi J, Lehtimäki T, Talvinen K, Raitakari OT, Vasankari T, Viikari J, Luoma J, Ylä-Herttuala S (1998) Baseline diene conjugation in LDL lipids as a direct measure of in vivo LDL oxidation. Clin Biochem 31:257–261
Esterbauer H, Gebicki J, Puhl H, Jürgens G (1992) The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med 13:341–390
Benzie IF, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76
Begic A et al (2017) The simple isocratic HPLC—UV method for the simultaneous determination of reduced and oxidized glutathione in animal tissue. Acta Chromatogr 29(1):67–84
Ceron JJ et al (2014) Serum paraoxonase 1 (PON1) measurement: an update. BMC Vet Res 10:74
H, A. (1984) Catalase in vitro. Methods Enzymol 105:121–129
Mazani M, Rezagholizadeh L, Shamsi S, Mahdavifard S, Ojarudi M, Salimnejad R et al (2022) Protection of CCl4-induced hepatic and renal damage by linalool. Drug Chem Toxicol 45(3):963–971
Kavita S, Neerja R, Khushboo G, Saurabh S (2017) Probable benefits of green tea with genetic implications. J Oral Maxillofac Pathol 21:107–114
Rabbani N, Thornalley PJ (2019) Glyoxalase 1 modulation in obesity and diabetes. Antioxid Redox Signal 20(3):354–374
Buraczynska M, Ksiazek K, Wacinski P, Zaluska W (2019) Interleukin-1β gene (IL1B) polymorphism and risk of develo** diabetic nephropathy. Immunol Investig 48(6):1–8
Song N, Thaiss F, Guo L (2019) NFκB and kidney injury. Front Immunol 16(10):815
Gao D, Madi M, Ding C, Fok M, Steele T, Ford C et al (2014) Interleukin-1beta mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am J Physiol Endocrinol 307:289–304
Giacco F, Du X, VD D’A, Milne R, Sui G, Geoffrion M, Brownlee M (2014) Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes 63(1):291–299
Ravarotto V, Bertoldi G, Innico G, Gobbi L, Calò LA (2021) The pivotal role of oxidative stress in the pathophysiology of cardiovascular-renal remodeling in kidney disease. Antioxidants 10(7):1041
Gyurászová M et al (2020) Oxidative stress in the pathophysiology of kidney disease: implications for noninvasive monitoring and identification of biomarkers. Oxidative Med Cell Longev 2020:5478708
Welbourne TC (1979) Ammonia production and glutamine incorporation into glutathione in the functioning rat kidney. Can J Biochem 57:233–237
Khatana C, Saini NK, Chakrabarti S, Saini V, Sharma A, Saini RV, Saini AK (2020) Mechanistic Insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxidative Med Cell Longev 15:2020
da Silva AA et al (2020) Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can J Cardiol 36(5):671–682
Whaley-Connell A, Sowers JR (2017) Insulin resistance in kidney disease: is there a distinct role separate from that of diabetes or obesity? Cardiorenal Med 8:41–49
Bjornstad P, Eckel RH (2018) Pathogenesis of lipid disorders in insulin resistance: a brief review. Curr Diab Rep 18(12):416–423
Cieslak MWA, C.M. (2015) Role of pro-inflammatory cytokines of pancreatic islets and prospects of elaboration of new methods for the diabetes treatment. Acta Biochim Pol 62(1):15–21
Vekic J, Vujcic S, Bufan B, Bojanin D, Al-Hashmi K, Al-Rasadi K, Stoian AP, Zeljkovic A, Rizzo M (2023) The role of advanced glycation end products on dyslipidemia. Metabolites 3(13):77
Acknowledgments
The results explained in this paper were part of the student thesis. The authors are thankful to Ardabil University of Medical Sciences for financial support.
Funding
This study is funded by Ardabil Medical Sciences University.
Author information
Authors and Affiliations
Contributions
(1) Study plan, evaluation, and exegesis of data: Mahdavifard and Najafzadeh. (2) Manuscript writing and revising: Mahdavifard. (3) Ultimate sanction of the version to be submitted and any revised version: Mahdavifard and Najafzadeh.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahdavifard, S., Nowruz, N. Glutamine Defended the Kidneys Versus Lead Intoxication Via Elevating Endogenous Antioxidants, Reducing Inflammation and Carbonyl Stress, as well as Improving Insulin Resistance and Dyslipidemia. Biol Trace Elem Res 202, 3141–3148 (2024). https://doi.org/10.1007/s12011-023-03887-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03887-7