Abstract
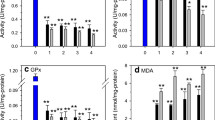
To investigate the protective effect of garlic powder on cadmium-induced toxicity sea bass liver, juvenile fishes where maintained under three food diets (diet 1: normal without garlic supply, diet 2: 2% garlic powder; diet 3: 6% garlic powder). After 30 days of specific diets, each group was injected with 500 μg kg−1of Cd. The control group was the one fed with normal diet and not injected with Cd. Liver Cd, Zn, and Se loads was assessed after 1 and 3 days of Cd injections. Moreover, antioxidant enzymes activities termed as catalase, superoxide dismutase, and glutathione peroxydase as well as their gene expression levels were monitored. Finally, metallothionein protein accumulation and its gene expression regulation (MTa) were determined. In fish fed with 2 and 6% garlic powder, the amounts of Cd, Zn, and Se significantly increase in liver tissues. Two percent garlic powder specific diet reversed the Cd-induced inhibition of catalase (CAT), superoxide dismutase (SOD), and gluthathione peroxydase (GPx) and restored the Cd-induced lipid peroxidation (MDA). The increase of liver metallothionein proteins as well as the MTa gene expression level under Cd influence was more pronounced in animals maintained for 30 days under garlic power 2% diet. Our data must be carefully considered in view of the garlic powder introduction in sea bass food composition at 2% since it is an efficient prevention against Cd-induced alterations.





Similar content being viewed by others
References
FAO (2016) The state of food and agriculture. Climate change agriculture and food security. FAO press. 194pp
Lalumera GM, Calamari D, Galli P, Castiglioni S, Crosa G, Fanelli R (2004) Preliminary investigation on the environmental occurrence and effects of antibiotics used in aquaculture in Italy. Chemosphere 54(5):661–668
Shi W, Deng D, Wang Y, Hu G, Guo J, Zhang X, Wang X, Giesy JP, Yu H, Wang Z (2016) Causes of endocrine disrupting potencies in surface water in East China. Chemosphere 144:1435–1442
Munday PL, Warner RR, Monro K, Pandolfi JM, Marshall DJ (2013) Predicting evolutionary responses to climate change in the sea. Ecol Lett 16(12):1488–1500
Fernández-Navarro M, Peragón J, Esteban FJ, de la Higuera M, Lupiáñez JA (2006) Maslinic acid as a feed additive to stimulate growth and hepatic protein-turnover rates in rainbow trout (Onchorhynchus mykiss). Comp Biochem Physiol C Toxicol Pharmacol. 144(2):130–140
Castro C, Peréz-Jiménez A, Coutinho F, Díaz-Rosales P, Serra CA, Panserat S, Corraze G, Peres H, Oliva-Teles A (2015) Dietary carbohydrate and lipid sources affect differently the oxidative status of European sea bass (Dicentrarchus labrax) juveniles. Br J Nutr 114(10):1584–1593
El-Barbary MI (2016) Detoxification and antioxidant effects of garlic and curcumin in Oreochromis niloticus injected with aflatoxin B1 with reference to gene expression of glutathione peroxidase (GPx) by RT-PCR. Fish Physiol Biochem 42(2):617–629
Citarasu T, Sivaram V, Immanuel G, Rout N, Murugan V (2006) Influence of selected Indian immunostimulant herbs against white spot syndrome virus (WSSV) infection in black tiger shrimp, Penaeus monodon with reference to haematological, biochemical and immunological changes. Fish Shellfish Immunol 21(4):372–384
Martins N, Petropoulos S, Ferreira IC (2016) Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: a review. Food Chem 211:41–50
Chi MS (1982) Effects of garlic products on lipid metabolism in cholesterol-fed rats (41494). Proc Soc Exp Biol Med 171(2):174–178
Banni M, Messaoudi I, Said L, El Heni J, Kerkeni A, Said K (2010) Metallothionein gene expression in liver of rats exposed to cadmium and supplemented with zinc and selenium. Arch Environ Contam Toxicol 59(3):513–519
Satarug S, Vesey DA, Gobe GC (2017) Current health risk assessment practice for dietary cadmium: data from different countries. Food Chem Toxicol 106(Pt A):430–445
Bouraoui Z, Banni M, Ghedira J, Clerandeau C, Guerbej H, Narbonne JF, Boussetta H (2008) Acute effects of cadmium on liver phase I and phase II enzymes and metallothionein accumulation on sea bream Sparus aurata. Fish Physiol Biochem 34(3):201–207
Banni M, Dondero F, Jebali J, Guerbej H, Boussetta H, Viarengo A (2007) Assessment of heavy metal contamination using real-time PCR analysis of mussel metallothionein mt10 and mt20 expression: a validation along the Tunisian coast. Biomarkers 12(4):369–383
Bouraoui Z, Banni M, Chouba L, Ghedira J, Clerandeau C, Jebali J, Narbonne JF, Boussetta H (2009) Monitoring pollution in Tunisian coasts using a scale of classification based on biochemical markers in worms Nereis (Hediste) diversicolor. Environ Monit Assess 164(1–4):691–700
Smaoui-Damak W, Rebai T, Berthet B, Hamza-Chaffai A (2006) Does cadmium pollution affect reproduction in the clam Ruditapes decussatus? A one-year case study. Comp Biochem Physiol C Toxicol Pharmacol. 143(2):252–261
Messaoudi I, Hammouda F, El Heni J, Baati T, Saïd K, Kerkeni A (2010) Reversal of cadmium-induced oxidative stress in rat erythrocytes by selenium, zinc or their combination. Exp Toxicol Pathol 62(3):281–288
Messaoudi I, El Heni J, Hammouda F, Saïd K, Kerkeni A (2009) Protective effects of selenium, zinc, or their combination on cadmium-induced oxidative stress in rat kidney. Biol Trace Elem Res 130(2):152–161
Thophon S, Kruatrachue M, Upatham ES, Pokethitiyook P, Sahaphong S, Jaritkhuan S (2003) Histopathological alterations of white seabass, Lates calcarifer, in acute and subchronic cadmium exposure. Environ Pollut 121(3):307–320
Amiard JC, Pineau A, Boiteau H-L, Metayer C, Amiard-Triquet C (1987) Application de la spectrométrie d’absorption atomique Zeeman aux dosages de huit éléments traces (Ag, Cd, Cr, Cu, Mn, Ni, Pb et Se) dans les matrices biologiques solides. Wat Res 21:693–697
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods in oxygen radical research. CRC Press, Florida, pp 283–294
Flohé L, Günzler WA (1984) Assay of glutathione peroxidase. Methods Enzymol 105:115–121
Crouch RK, Gandy SC, Kinsey G (1981) The inhibition of islet superoxide dismutase by diabetogenic drugs. Diabetes 30:235–241
Buege JA, Aust SD (1978) Microsomal lipid peroxidation, in: L. Packer (Ed.), Meth. Enzymol. 52, 302–310
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Mar Environ Res 44:69–84
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–169
Banni M, Hajer A, Sforzini S, Oliveri C, Boussetta H, Viarengo A (2014) Transcriptional expression levels and biochemical markers of oxidative stress in Mytilus galloprovincialis exposed to nickel and heat stress. Comp Biochem Physiol C Toxicol Pharmacol 160:23–29
Dondero F, Banni M, Negri A, Boatti L, Dagnino A, Viarengo A (2011) Interactions of a pesticide/heavy metal mixture in marine bivalves: a transcriptomic assessment. BMC Genomics 16:12–195
Negri A, Oliveri C, Sforzini S, Mignione F, Viarengo A, Banni M (2013) Transcriptional response of the mussel Mytilus galloprovincialis (Lam.) following exposure to heat stress and copper. PLoS One. https://doi.org/10.1371/journal.pone.0066802
Dondero F, Piacentini L, Banni M, Rebelo M, Burlando B, Viarengo A (2005) Quantitative PCR analysis of two molluscan metallothionein genes unveils differential expression and regulation. Gene 345:259–270
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Research 30:e36
Waisberg M, Joseph P, Hale B, Beyersmann D (2003) Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 192(2–3):95–117
Mohamed EH, Baiomy AA, Ibrahim ZS, Soliman MM (2016) Modulatory effects of levamisole and garlic oil on the immune response of Wistar rats: biochemical, immunohistochemical, molecular and immunological study. Mol Med Rep 14(3):2755–2763
Ziamajidi N, Nasiri A, Abbasalipourkabir R, Sadeghi MS (2017) Effects of garlic extract on TNF-α expression and oxidative stress status in the kidneys of rats with STZ + nicotinamide-induced diabetes. Pharm Biol 55(1):526–531
Nemmiche S (2017) Oxidative signaling response to cadmium exposure. Toxicol Sci 156(1):4–10
Alkharashi NAO, Periasamy VS, Athinarayanan J, Alshatwi AA (2017) Cadmium triggers mitochondrial oxidative stress in human peripheral blood lymphocytes and monocytes: analysis using in vitro and system toxicology approaches. J Trace Elem Med Biol 42:117–128
Stohs SJ, Bagachi C (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336
Casalino E, Cesare S, Clemente L (1997) Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Arch Biochem Biophys 346:171–179
Price-Haughey J, Bonham K, Gedamu L (1986) Heavy metal-induced gene expression in fish and fish cell lines. Environ Health Perspect 65:141–147
Thiele DJ (1992) Metal-regulated transcription in eukaryotes. Nucl Acid Resear 20:1183–1191
Tohyama C, Satoh M, Kodama N, Nishimura H, Choo A, Michalska A, Kanayama Y, Naganuma A (1996) Reduced retention of cadmium in the liver of metallothionein-null mice. Environ Toxicol Pharmacol 1:213–216
Sadhu C, Gedamu L (1988) Regulation of human metallothionein (MT) genes. Differential expression of MTI-F, MTI-G, and MTII-A genes in the hepatoblastoma cell line (HepG2). J Biol Chem 263:2679–2684
Acknowledgments
This work was supported by funds from the Ministry of Scientific Research and Technology, Tunisia (Unité de Recherche en Biochimie et Toxicologie Environnementale), ISA Chott-Mariem.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Mosbah, A., Guerbej, H., Boussetta, H. et al. Protective Effects of Dietary Garlic Powder Against Cadmium-induced Toxicity in Sea Bass Liver: a Chemical, Biochemical, and Transcriptomic Approach. Biol Trace Elem Res 183, 370–378 (2018). https://doi.org/10.1007/s12011-017-1146-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1146-4