Abstract
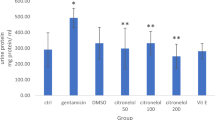
The aim of this study was to investigate the effect of extracorporeal shock wave lithotripsy (ESWL) on kidney oxidative stress and trace element levels of adult rats. Twelve male Wistar albino rats were divided equally into two groups. First group was used as control. The right-side kidneys of animals in second group were treated with 2,000 18-kV shock waves under anesthesia. Localization of the right kidney was achieved following contrast medium injection through a tail vein under flouroscopy control. The animals were sacrificed 72 h after the ESWL treatment, and the kidneys were taken. Malondialdehyde level was higher in the ESWL group than in the control. Reduced glutathione levels, superoxide dismutase, and glutathione peroxidase activities were lower in the ESWL group than those of the control. Fe, Cu, Pb, Mn, Cd, and Ni levels were lower in the ESWL group than in the control, although Mg level was higher in the ESWL group than in the control. In conclusion, the result of the present study indicated that ESWL treatment produced oxidative stress in the kidney and caused impairments on the antioxidant and trace element levels in the kidneys of rats.




Similar content being viewed by others
Abbreviations
- ESWL:
-
Extracorporeal shock wave lithotripsy
References
Serel TA, Ozguner F, Soyupek S (2004) Prevention of shock wave-induced renal oxidative stress by melatonin: an experimental study. Urol Res 32:69–71
Morgan TR, Laudone PV, Heston WD, Zeitz L, Fair WR (1988) Free radical production by high energy shock waves comparison with ionizing irradiation. J Urol 139:186–189
Suhr D, Brummer F, Hulcer DF (1991) Cavitation-generated free radicals during shock wave exposure: investigations with cell-free solutions and suspended cells. Ultrasound Med Biol 17:761–768
Sokolov DL, Bailey MR, Crum LA, Blomgren PM, Connors BA, Evan AP (2002) Prefocal alignment improves stone comminution in shockwave lithotripsy. J Endourol 16:709–715
Kırkali Z, Kırkali G, Tahiri Y (1994) The effect of extracorporeal electromagnetic shock waves on renal proximal tubular function. Int Urol Nephrol 26:255–257
Crum LA (1988) Cavitation microjets as a contributory mechanism for renal calculus disintegration in ESWL. J Urol 140:1587–1590
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Kökçam İ, Nazıroğlu M (1999) Antioxidant and lipid peroxidation status in the blood of patients with psoriasis. Clin Chim Acta 289:23–31
Newman R, Hackett R, Senior D, Brock K, Feldman J, Sosnowski J, Finlayson B (1987) Pathologic effects of ESWL on canine renal tissue. Urology 29:194–200
Karalezli G, Goğuş O, Beduk Y, Kokuuslu C, Sarıca K, Kutsal O (1993) Histopathologic effects of extracorporeal shock wave lithotripsy on rabbit kidney. Urol Res 21:67–70
Turgut M, Unal I, Berber A, Demir TA, Mutlu F, Aydar Y (2008) The concentration of Zn, Mg and Mn in calcium oxalate monohydrate stones appears to interfere with their fragility in ESWL therapy. Urol Res 36:31–38
Markovich D (2011) Invited Review—Physiological roles of renal anion transporters Nas1 and Sat1. Am J Physiol Renal Physiol 94(5):562–573
Placer ZA, Cushman L, Johnson BC (1966) Estimation of products of lipid peroxidation (malonyl dialdehyde) in biological fluids. Anal Biochem 16:359–364
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–69
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Aebi H (1974) Catalase. In: Bergmayer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 673–677
Sedlak J, Lindsay RHC (1968) Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellmann’s reagent. Anal Biochem 25:192–205
Li X, He D, Zhang L, Cheng X, Sheng B, Luo Y (2006) A novel antioxidant agent, astragalosides, prevents shock wave-induced renal oxidative injury in rabbits. Urol Res 34:277–282
Sheng B, He D, Zhao J, Chen X, Nan X (2011) The protective effects of the traditional Chinese herbs against renal damage induced by extracorporeal shock wave lithotripsy: a clinical study. Urol Res 39:89–97
Sarica K, Koşar A, Yaman O, Bedük Y, Durak I, Göğüş O, Kavukçu M (1996) Evaluation of ischemia after ESWL: detection of free oxygen radical scavenger enzymes in renal parenchyma subjected to high-energy shock waves. Urol Int 57:221–223
Aksoy Y, Malkoc İ, Atmaca AF, Aksoy H, Altinkaynak K, Akcay F (2006) The effects of extracorporeal shock wave lithotripsy on antioxidant enzymes in erythrocytes. Cell Biochem Funct 24:467–469
Munver R, Delvecchio FC, Kuo RL, Brown SA, Zhong P, Preminger GM (2002) In vivo assessment of free radical activity during shock wave lithotripsy using a microdialysis system: the renoprotective action of allopurinol. J Urol 167:327–334
Nazıroğlu M (2009) Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Aggett PJ (1999) An overview of the metabolism of copper. Eur J Med Res 4:214–216
Ertekin A, Değer Y, Mert H, Mert N, Yur F, Dede S, Demir H (2006) An investigation of the effects of alpha-tocopherol on the levels Fe, Cu, Zn, Mn and carbonic anhydrase in rats with bleomycin-induced pulmonary fibrosis. Biol Trace Elem Res 114:1–12
Özkaya MO, Nazıroğlu M, Barak C, Berkkanoğlu M (2011) Effects of multivitamin/mineral supplementation on trace element levels in serum and follicular fluid of women undergoing in vitro-fertilization (IVF). Biol Trace Elem Res 139:1–9
Kayan M, Nazıroğlu M, Barak C (2010) Effects of vitamin C and E combination on trace element levels in blood of smokers and nonsmokers radiology X-ray technicians. Biol Trace Elem Res 136:140–148
Lustberg M, Silbergeld E (2002) Blood lead levels and mortality. Arch Intern Med 162:2443–2449
Acknowledgments
We thank Dr. Sıdık Keskin for his help in statistical evaluation of our data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gecit, İ., Kavak, S., Meral, I. et al. Effects of Shock Waves on Oxidative Stress, Antioxidant Enzyme and Element Levels in Kidney of Rats. Biol Trace Elem Res 144, 1069–1076 (2011). https://doi.org/10.1007/s12011-011-9124-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9124-8