Abstract
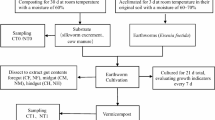
The ideal condition of earthworm gut promotes growth and multiplication of beneficial soil microorganisms eliminating pathogens and converts organic wastes into nutrients rich compost. The present study has been carried out to determine the population dynamics of earthworm gut bacteria and to find out relative abundance of different functional bacterial groups in the foregut, midgut, and hindgut of earthworm Perionyx excavatus. To assess bacterial diversity, a viable plate count method was adopted. In the different gut region of earthworm, aerobic heterotrophic, amylolytic, Bacillus, Gram-negative, proteolytic, fat hydrolyzing, nitrate-reducing, nitrifying, asymbiotic nitrogen-fixing, Azotobacter, and phosphate solubilizing bacterial populations ranged from 22.2 to 241.6 × 106, 8.0 to 171.60 × 106, 1.83 to 2.79 × 106, 10.68 to 23.04 × 104, 3.70 to 5.52 × 104, 59.60 to 208.40 × 104, 1.86 to 7.34 × 104, 10.94 to 19.78 × 104, 0.80 to 3.42 × 104, 7.83 to 13.70 × 104, 1.31 to 2.67 × 104 cfu/ml gut suspension, respectively. The results of the one-way ANOVA revealed that the bacterial load of most of the bacterial groups was significantly higher (p < 0.05) in the hindgut region, followed by midgut and foregut. Only the density of the proteolytic group was significantly higher (p < 0.05) in the midgut region followed by foregut and hindgut. Starch hydrolyzing bacteria constitute the largest group of bacteria in the gut content. From principal component analysis, two components were extracted with the eigenvalues of 8.485 and 1.132. Agglomerative hierarchical cluster analysis revealed that the bacterial populations were clustered into four different groups. Quantitative variation among bacterial groups in earthworm’s gut seems to determine the soil health and composting efficiency; from this point of view, the present study will provide a better understanding about different functional bacterial groups of earthworm’s guts and might be helpful in sustainable agriculture and waste management.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Zhang, W., Dima, C., & Cancan, Z. (2007). Functions of earthworm in ecosystem. Biodiversity Science, 15(2), 142.
Horn, M. A., Drake, H. L., & Schramm, A. (2006). Nitrous oxide reductase genes (nosZ) of denitrifying microbial populations in soil and the earthworm gut are phylogenetically similar. Applied and Environment Microbiology, 72(2), 1019–1026.
Nataraj, B., Hemalatha, D., Rangasamy, B., Maharajan, K., & Ramesh, M. (2017). Hepatic oxidative stress, genotoxicity and histopathological alteration in fresh water fish Labeo rohita exposed to organophosphorus pesticide profenofos. Biocatalysis and Agricultural Biotechnology, 12, 185–190. https://doi.org/10.1016/j.bcab.2017.09.006
Majumder, R., & Kaviraj, A. (2019). Acute and sublethal effects of organophosphate insecticide chlorpyrifos on freshwater fish Oreochromis niloticus. Drug and Chemical Toxicology, 42, 487–495. https://doi.org/10.1080/01480545.2018.1425425
Sinha, R. K., Agarwal, S., Chauhan, K., & Valani, D. (2010). The wonders of earthworms & its vermicompost in farm production: Charles Darwin’s ‘friends of farmers’, with potential to replace destructive chemical fertilizers. Agricultural Science, 01(02), 76–94. https://doi.org/10.4236/as.2010.12011
Ravindran, B., Contreras-Ramos, S. M., & Sekaran, G. (2015). Changes in earthworm gut associated enzymes and microbial diversity on the treatment of fermented tannery waste using epigeic earthworm Eudrilus eugeniae. Ecological Engineering, 74, 394–401.
Chao, H. L., Kong, H., Zhang, M., Sun, M., Ye, D., Huang, Z., Zhang, D., Sun, S., Zhang, Y., Yuan, M., Liu, M., Hu, F., & Jiang, X. (2019). Metaphire guillelmi gut as hospitable micro-environment for the potential transmission of antibiotic resistance genes. Science of The Total Environment, 669, 353–361.
Drake, H. L., & Horn, M. A. (2007). As the worm turns: The earthworm gut as a transient habitat for soil microbial biomes. Annual Review of Microbiology, 61, 169–189.
Edwards, C. A. , Domínguez, J. & Arancon, N. Q. (2004). The influence of vermicomposts on plant growth and pest incidence. In: S. H., Shakir & W. Z. A., Mikhail (Eds.), Soil Zoology for Sustainable Development in the 21st Century, Cairo, pp 397–420. http://jdguez.webs.uvigo.es/wp-content/uploads/2011/10/The-influence-of-vermicompost-on-plant-growth-and-pest-incidence.pdf
Munnoli, M. P. (2007). Management of industrial organic solid waste through vermi-culture biotechnology with special reference to microorganisms. PhD diss., Goa University, India. http://hdl.handle.net/10603/12461. Accessed 19 Nov 2021.
Govindarajan, V. & Prabaharan. (2014). Gut micro-floral of earthworms: a review. American Journal of Biological and Pharmaceutical Research, 1(3), 125–130. https://www.researchgate.net/publication/265595977_GUT_MICRO-FLORA_OF_EARTHWORMS_A_REVIEW
Lv, B., **ng, M., & Yang, J. (2018). Exploring the effects of earthworms on bacterial profiles during vermicomposting process of sewage sludge and cattle dung with high-throughput sequencing. Environmental Science and Pollution Research, 25, 12528–12537. https://doi.org/10.1007/s11356-018-1520-6
Horn, A. M., Andreas, S., & Harold, L. D. (2003). The earthworm gut: An ideal habitat for ingested N2O-producing microorganisms. Applied and Environment Microbiology, 69(3), 1662–1669.
Villar, D., Alves, D., Pérez-Díaz, S., & Mato,. (2016). Changes in microbial dynamics during vermicomposting of fresh and composted sewage sludge. Waste Management, 48, 409–417. https://doi.org/10.1016/j.wasman.2015.10.011
Liu, D. F., Lian, B., & Wang, B. (2016). Solubilization of potassium containing minerals by high temperature resistant Streptomyces sp isolated from earthworm’s gut. Acta Geochimica, 35(3), 262–270.
Kiyasudeen, K., Ibrahim, M. H., Quaik, S., & Ismail, S. A. (2015). Prospects of organic waste management and the significance of earthworms. Springer.
Pathma, J., & Sakthivel, N. (2013). Molecular and functional characterization of bacteria isolated from straw and goat manure based vermicompost. Applied Soil Ecology, 70, 33–47. https://doi.org/10.1016/j.apsoil.2013.03.011
Parle, J. N. (1963). Micro-organisms in the intestines of earthworms. Microbiology, 31(1), 1–11.
Ranganathan, L. S., & Vinotha, S. P. (1998). Influence of pressmud on the enzymatic variations in the different reproductive stages of Eudrilus eugeniae (Kinberg). Current Science, 74(7), 634–635.
Tracey, M. V. (1951). Cellulase and chitinase of earthworms. Nature, 167(4254), 776–777.
Edwards, C. A., & Fletcher, K. E. (1988). Interactions between earthworms and microorganisms in organic-matter breakdown. Agriculture, Ecosystems & Environment, 24(1–3), 235–247.
Pramanik, P., Ghosh, G. K., Ghosal, P. K., & Banik, P. (2007). Changes in organic–C, N, P and K and enzyme activities in vermicompost of biodegradable organic wastes under liming and microbial inoculants. Bioresource Technology, 98(13), 2485–2494.
Koubová, A., Chroňáková, A., Pižl, V., Sánchez-Monedero, M. A., & Elhottová, D. (2015). The effects of earthworms Eisenia spp. on microbial community are habitat dependent. European Journal of Soil Biology, 68, 42–55.
Zhu, B. K., Fang, Y. M., Zhu, D., Christie, P., Ke, X., & Zhu, Y. G. (2018). Exposure to nanoplastics disturbs the gut microbiome in the soil oligochaete Enchytraeus crypticus. Environmental Pollution, 239, 408–415.
Liu, D. F., Lian, B., Wu, C., & Guo, P. (2018). A comparative study of gut microbiota profiles of earthworms fed in three different substrates. Symbiosis, 74(1), 21–29.
Pelczar, M. J., Bard, R. C., Burnett, G. W., Conn, H. J., Demoss, R. D., Euans, E. E., Weiss, F. A., Jennison, M. W., Meckee, A. P., Riker, A. J., Warren, J. & Weeks, O. B. (1957). Manual of microbiological methods, Society of American Bacteriology. McGraw Hill Book Company Inc.
Lacey, L. A. ed. (1997). Manual of techniques in insect pathology. Academic Press.
Zar, J. H. (1999). Biostatistical analysis. Pearson Education India.
Gniazdowski, Z. (2017). New interpretation of principal components analysis. ar**v preprint ar**v, 1711, 10420.
Martin, A., Cortez, J., Barosis, I., & Lavelle, P. (1987). Les mucus intestinaux de Var de Terre, moteur de leurs interactions avec la microflore. Revue d’Écologie et de Biologie du Sol, 24, 549–558.
Barois, I., & Lavelle, P. (1986). Changes in respiration rate and some physico-chemical properties of a tropical soil during transit through Pontoscolex corethrurus (Glossoscolecidae, Oligochaeta). Soil Biology & Biochemistry, 18, 539–541.
Idowu, A. B., Edema, M. O., & Adeyi, A. O. (2006). Distribution of bacteria and fungi in the earthworm Libyodrillus violaceous (Annelida: Oligochaeta), a native earthworm from Nigeria. Revista de Biologia Tropical, 54(1), 49–58.
Birundha, M., Paul, J. J., & Mariappan, P. (2013). Growth and reproduction of Perionyx excavatus in different organic wastes. International Journal of Current Microbiology and Applied Sciences, 2(2), 28–35.
Stockli, A. (1928). Studien tiber den Einfluss der Regenwtirmer auf die Beschaffenheit des Bodens. Landwirtsch Jahrb Schweiz, 42, 1–121.
Chowdhury, A., Hazra, A. K., Mahajan, S. & Choudhury, J. (2007). Microbial communities of earthworm (Perionyx excavatus Perrier) gut, cast and adjacent Soil in two different fields of west Bengal. Rec. Zoo. Surv. India. l07(Part-4), 101–113. http://faunaofindia.nic.in/PDFVolumes/records/107/04/0101-0113.pdf
Kristufek, V., Ravasz, K., & Pizl, V. (1992). Changes in densities of bacteria and microfungi during gut transit in Lumbricus rubellus and Aporrectodea caliginosa (Oligochaeta: Lumbricidae). Soil Biology & Biochemistry, 24, 1499–1500.
Valle-Molinares, R., Borges, S., & Rios-Velazquez, C. (2007). Characterization of possible symbionts in Onychochaeta borincana (Annelida: Glossoscolecidae). European Journal of Soil Biology, 43, S14–S18.
Mishra, P. C., & Dash, M. C. (1980). Digestive enzymes of some earthworms. Experientia, 36(10), 1156–1157.
Kim, H. J., Shin, K. H., Cha, J. C., & Hur, H. G. (2004). Analysis of aerobic and culturable bacterial community structures in earthworm (Eisenia fetida) intestine. Journal of Applied Biological Chemistry, 47(3), 137–142.
Ponomareva, S. I. (1953). The influence of the activity of earthworms on the creation of a stable structure in a sod-podzolized soil. Tr Pochv Inst Dokuchaeva., 41, 304–378.
Ghosh, S., & Chatterjee, S. (2020). Isolation and characterization of Bacillus tequilensis from gut content of Perionyx excavatus and evaluation of its starch hydrolyzing property. Biosc. Biotech. Res. Comm., 13, 670–675. https://doi.org/10.21786/bbrc/13.2/45
Benitez, E., Nogales, R., Elvira, C., Masciandaro, G. & Ceccanti, B. (1999). Enzyme and earthworm activities during vermicomposting of carbaryl-treated sewage sludge. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, 28(4), 1099–1104. https://doi.org/10.2134/jeq1999.00472425002800040006x
Zhou, G. W., Yang, X. R., Sun, A. Q., Li, H., Lassen, S. B., Zheng, B. X., & Zhu, Y. G. (2019). Mobile incubator for iron (III) reduction in the gut of the soil-feeding earthworm Pheretima guillelmi and interaction with denitrification. Environmental Science and Technology, 53(8), 4215–4223.
Sun, M., Chao, H., Zheng, X., Deng, S., Ye, M. & Hu, F. (2020). Ecological role of earthworm intestinal bacteria in terrestrial environments: a review. Science Total Environment, p.140008. https://doi.org/10.1016/j.scitotenv.2020.140008
Wang, H. T., Zhu, D., Li, G., Zheng, F., Ding, J., O’Connor, P. J., Zhu, Y. G., & Xue, X. M. (2019). Effects of arsenic on gut microbiota and its biotransformation genes in earthworm Metaphire sieboldi. Environmental Science and Technology, 53(7), 3841–3849.
Acknowledgements
The authors are grateful to WBDST, DST-FIST, and DST-PURSE for providing instrumental facilities. The authors are thankful to the Head, Department of Zoology, and the authority of The University of Burdwan for giving all sorts of laboratory facilities to conduct this research.
Author information
Authors and Affiliations
Contributions
Sucharita Ghosh: conceptualization, validation, statistical analysis, data curation, methodology, and writing original draft. Soumendranath Chatterjee: study design, conceptualization, data curation, writing, review, editing, visualization, and supervision. Dipanwita Sarkar Paria: conceptualization, writing, review, editing, and visualization.
Corresponding author
Ethics declarations
Ethics approval
Proper approval from Institutional Biosafety Committee was obtained at starting of the research work.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghosh, S., Sarkar Paria, D. & Chatterjee, S. Comparative Study on Bacterial Population Dynamics of Foregut, Midgut, and Hindgut Content of Perionyx excavatus (Perrier) Isolated from Eco-friendly, Non-hazardous Vermicompost. Appl Biochem Biotechnol 194, 6126–6139 (2022). https://doi.org/10.1007/s12010-022-03970-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03970-0