Abstract
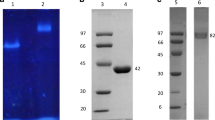
Aspergillus niger has been used for homologous and heterologous expressions of many protein products. In this study, the α-L-rhamnosidase from A. niger (Rha-N1, GenBank XP_001389086.1) was homologously expressed in A. niger 3.350 by Agrobacterium tumefaciens-mediated transformation. The enzyme activity of Rha-N1 was 0.658 U/mL, which was obtained by cultivation of engineered A. niger in a 5-L bioreactor. Rha-N1 was purified by affinity chromatography and characterized. The optimum temperature and optimum pH for Rha-N1 were 60 °C and 4.5, respectively. Enzyme activity was promoted by Al3+, Li+, Mg2+, and Ba2+ and was inhibited by Mn2+, Fe3+, Ca2+, Cu2+, and organic solvents. The result indicated that rutin was the most suitable substrate for Rha-N1 by comparison with the other two flavonoid substrates hesperidin and naringin. The transformed products of isoquercitrin, hesperetin-7-O-glucoside, and prunin were identified by LC–MS and 1H-NMR.







Similar content being viewed by others
Data Availability
Data are available on request.
References
Carceller, J. M., Martínez Galán, J. P., Monti, R., Bassan, J. C., Filice, M., Iborra, S., Yu, J., & Corma, A. (2019). Selective synthesis of citrus flavonoids prunin and naringenin using heterogeneized biocatalyst on graphene oxide. Green Chemistry, 21, 839–849.
Céliz, G., Rodriguez, J., Soria, F., & Daz, M. (2015). Synthesis of hesperetin 7-O-glucoside from flavonoids extracted from Citrus waste using both free and immobilized α-l-rhamnosidases. Biocatalysis and Agricultural Biotechnology, 4, 335–341.
Eom, S. J., Kim, K. T., & Paik, H. D. (2018). Microbial bioconversion of ginsenosides in Panax ginseng and their improved bioactivities. Food Reviews International, 34, 698–712.
Resham, K., Khare, P., Bishnoi, M., & Sharma, S. S. (2020). Neuroprotective effects of isoquercitrin in diabetic neuropathy via Wnt/β-catenin signaling pathway inhibition. BioFactors (Oxford), 46, 411–420.
Biswas, T., Mathur, A. K., & Mathur, A. (2017). A literature update elucidating production of Panax ginsenosides with a special focus on strategies enriching the anti-neoplastic minor ginsenosides in ginseng preparations. Applied Microbiology and Biotechnology, 101, 4009–4032.
Zhao, Y., & Li, C. (2018). Biosynthesis of plant triterpenoid saponins in microbial cell factories. Journal of Agricultural and Food Chemistry, 66, 12155–12165.
Yadav, S., Yadav, R. S. S., & Yadav, K. D. S. (2013). An α-l-rhamnosidase from Aspergillus awamori MTCC-2879 and its role in debittering of orange juice. International Journal of Food Science & Technology, 48, 927–933.
Zhang, R., Zhang, B. L., Li, G. C., **e, T., Hu, T., & Luo, Z. Y. (2015). Enhancement of ginsenoside Rg(1) in panax ginseng hairy root by overexpressing the alpha-l-rhamnosidase gene from Bifidobacterium breve. Biotechnology Letters, 37, 2091–2096.
Bourbouze, R., Pratviel-Sosa, F., & Percheron, F. (1975). Rhamnodiastase et α-l-rhamnosidase de fagopyrum esculentum. Phytochemistry (Oxford), 14, 1279–1282.
Suzuki, H. (1962). Hydrolysis of flavonoid glycosides by enzymes (rhamnodiastase) from rhamnus and other sources. Archives of Biochemistry and Biophysics, 99, 476–483.
Thomas, D. W., Smythe, C. V., & Labbee, M. D. (1958). Enzymatic hydrolysis of naringin, the bitter principle of grapefruit. Journal of Food Science, 23, 591–598.
Hall, D. H. (1938). A new enzyme of the glycosidase type. Nature (London), 142, 150–150.
Antón-Millán, N., García-Tojal, J., Marty-Roda, M., Garroni, S., Cuesta-López, S., & Tamayo-Ramos, J. A. (2018). Influence of three commercial graphene derivatives on the catalytic properties of a Lactobacillus plantarum α-l-rhamnosidase when used as immobilization matrices. ACS Applied Materials & Interfaces, 10, 18170–18182.
Avila, M., Jaquet, M., Moine, D., Requena, T., Peláez, C., Arigoni, F., & Jankovic, I. (2009). Physiological and biochemical characterization of the two α-l-rhamnosidases of Lactobacillus plantarum NCC245. Microbiology (Society for General Microbiology), 155, 2739–2749.
De Winter, K., Šimčíková, D., Schalck, B., Weignerová, L., Pelantova, H., Soetaert, W., Desmet, T., & Křen, V. (2013). Chemoenzymatic synthesis of α-l-rhamnosides using recombinant α-l-rhamnosidase from Aspergillus terreus. Bioresource Technology, 147, 640–644.
Manzanares, P., de Graaff, L. H., & Visser, J. (1997). Purification and characterization of an α-l-rhamnosidase from Aspergillus niger. FEMS Microbiology Letters, 157, 279–283.
Yadav, S., Yadav, V., Yadava, S., & Yadav, K. D. S. (2012). Purification and functional characterisation of an α-l-rhamnosidase from Penicillium citrinum MTCC-3565. International Journal of Food Science & Technology, 47, 1404–1410.
Han, B., & Fu, S. P. (2008). Characterization of glycoside-α-rhamnosidase from Absidia sp. Journal of Dalian Polytechnic University, 27, 105–109.
Yadav, V., Yadav, P. K., Yadav, S., & Yadav, K. D. S. (2010). α-l-Rhamnosidase: A review. Process Biochemistry, 45, 1226–1235.
Zverlov, V. V., Hertel, C., Bronnenmeier, K., Hroch, A., Kellermann, J., & Schwarz, W. H. (2000). The thermostable α-l-rhamnosidase RamA of Clostridium stercorarium: Biochemical characterization and primary structure of a bacterial α-l-rhamnoside hydrolase, a new type of inverting glycoside hydrolase. Molecular Microbiology, 35, 173–179.
Wang, D. Q., Zheng, P., & Chen, P. C. (2019). Production of a recombinant α-l-rhamnosidase from Aspergillus niger CCTCC M 2018240 in Pichia pastoris. Applied Biochemistry and Biotechnology, 189, 1020–1037.
Fleißner, A., & Dersch, P. (2010). Expression and export: Recombinant protein production systems for Aspergillus. Applied Microbiology and Biotechnology, 87, 1255–1270.
Meyer, V., Wu, B., & Ram, A. F. J. (2010). Aspergillus as a multi-purpose cell factory: Current status and perspectives. Biotechnology Letters, 33, 469–476.
Punt, P. J., van Biezen, N., Conesa, A., Albers, A., Mangnus, J., & van den Hondel, C. (2002). Filamentous fungi as cell factories for heterologous protein production. Trends In Biotechnology, 20, 200–206.
Ward, O. P. (2012). Production of recombinant proteins by Filamentous fungi: Production of recombinant proteins. Biotechnology Advances, 30, 1119–1139.
Pel, H. J., Winded, J. H., Archer, D. B., Dyer, P. S., & Hofmann, G. (2007). Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nature Biotechnology, 25, 221–231.
Poturcu, K., Ozmen, I., & Biyik, H. H. (2016). Characterization of an alkaline thermostable pectin lyase from newly isolated Aspergillus niger WHAK1 and its application on fruit juice clarification. Arabian Journal for Science and Engineering, 42, 19–29.
Cai, L. N., Xu, S. N., Lu, T., Lin, D. Q., & Yao, S. J. (2019). Directed expression of halophilic and acidophilic β-glucosidases by introducing homologous constitutive expression cassettes in marine Aspergillus niger. Journal of Biotechnology, 292, 12–22.
Han, G., Shao, Q., Li, C., Zhao, K., Jiang, L., Fan, J., Jiang, H., & Tao, F. (2018). An efficient Agrobacterium-mediated transformation method for aflatoxin generation fungus Aspergillus flavus. The Journal of Microbiology, 56, 356–364.
Michielse, C. B., Hooykaas, P. J., Hondelvd, C. A., & Ram, A. F. (2005). Agrobacterium-mediated transformation as a tool for functional genomics in fungi. Current Genetics, 48, 1–17.
Michielse, C. B., Hooykaas, P. J. J., van den Hondel, C. A. M. J. J., & Ram, A. F. J. (2008). Agrobacterium-mediated transformation of the filamentous fungus Aspergillus awamori. Nature Protocols, 3, 1671–1678.
Sun, Y., Niu, Y., He, B., Ma, L., Li, G., Tran, V. T., Zeng, B., & Hu, Z. (2019). A dual selection marker transformation system using Agrobacterium tumefaciens for the industrial Aspergillus oryzae 3.042. Journal of Microbiology and Biotechnology, 29, 230–234.
Yadav, V., Yadav, S., Yadava, S., & Yadav, K. D. S. (2012). α-L-rhamnosidase from Aspergillusclavato-nanicus MTCC-9611 active at alkaline pH. Applied Biochemistry and Microbiol, 48, 295–301.
Yadav, S., Yadava, S., & Yadav, K. D. S. (2013). Purification and characterization of α-L-rhamnosidase from Penicillium corylopholum MTCC-2011. Process Biochemistry, 48(9), 1348–1354.
Kumar, D., Yadav, S., Yadava, S., & Yadav, K. D. S. (2019). An alkali tolerant α-l-rhamnosidase from Fusarium moniliforme MTCC-2088 used in de-rhamnosylation of natural glycosides. Bioorganic Chemistry, 84, 24–31.
Kristína, M., Lenka, W., Michal, R., Vladimír, K. & Martin, R. (2015). Upscale of recombinant α-L-rhamnosidase production by Pichia pastoris MutS strain. Frontiers in Microbiology, 6.
Ge, L., & **e, J. (2017). Purification and characterisation of a novel α-L-rhamnosidase exhibiting transglycosylating activity from Aspergillus oryzae. International Journal of Food Science & Technology, 52(12), 2596–2603.
Yadav, S., & Yadava, S. (2017). α-L-Rhamnosidase selective for rutin to isoquercitrin transformation from Penicillium griseoroseum MTCC-9224. Bioorganic Chemistry, 70, 222–228.
Zhang, T., & Yuan, W. (2018). Purification and characterization of an intracellular α-L-rhamnosidase from a newly isolated strain, Alternaria alternata SK37. 001. Food Chemistry, 269, 63–69.
Tian, J., Wang, P., Huang, L., Chu, X., Wu, N., & Fan, Y. (2013). Improving the thermostability of methyl parathion hydrolase from Ochrobactrum sp. M231 using a computationally aided method. Applied Microbiology and Biotechnology, 97, 2997–3006.
Li, L., Gong, J., Wang, S., Li, G., Gao, T., Jiang, Z., Cheng, Y. S., Ni, H., & Li, Q. (2019). Heterologous expression and characterization of a new clade of Aspergillus α-L-rhamnosidase suitable for citrus juice processing. Journal of Agricultural and Food Chemistry, 67, 2926–2935.
de Lise, F., Mensitieri, F., & Tarallo, V. (2016). RHA-P: Isolation, expression and characterization of a bacterial a-l-rhamnosidase from Novosphingobium sp. PP1Y. Journal of Molecular Catalysis B: Enzymatic, 134, 136–147.
Funding
This research was financially supported by the National Natural Science Foundation of China (31600639), Science and Technology Project Founded by the Education Department of Jiangxi Province (GJJ202305), and Zhejiang University Student Science and Technology Innovation Activity Plan (**nMiao Talent Plan, 2021R403094).
Author information
Authors and Affiliations
Contributions
Jianyong Zheng and **aojun Li contributed to conceptualization; Hangyu Ye and **aojun Li performed data curation; Jianyong Zheng, **aojun Li, and Hangyu Ye contributed to funding acquisition; Hangyu Ye, Luyuan Li, and Yinjun Zhang performed investigation; Jianyong Zheng and **aojun Li were involved in supervision; Hangyu Ye and **aojun Li performed writing original draft.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
The final manuscript has been seen and approved by all the authors.
Consent to Publish
All the authors mutually agreed that the work should be published in Applied Biochemistry and Biotechnology.
Competing Interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ye, H., Li, X., Li, L. et al. Homologous Expression and Characterization of α-L-rhamnosidase from Aspergillus niger for the Transformation of Flavonoids. Appl Biochem Biotechnol 194, 3453–3467 (2022). https://doi.org/10.1007/s12010-022-03894-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03894-9