Abstract
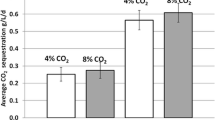
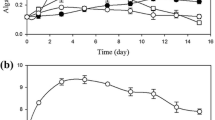
The effects of exogenous CO2 on the growth and lipid accumulation of a local screened facultative heterotrophic microalgae strain Auxenochlorella protothecoides (UMN280) as well as nutrient removal from concentrated municipal wastewater stream (centrate) were examined in this study. A 12-day batch experiment was conducted with CO2 aeration at three levels, namely, 0%, 1%, and 5% (v/v) CO2 mixed with air, under light intensity of 60 μmol/(m2 @@s). A two-stage growth pattern was observed. The first stage (first–fifth day) was dominated by heterotrophic growth in which organic carbon was the main carbon source. The second stage (6th–12th day) was dominated by autotrophic growth in which exogenous CO2 had a positive effect on algal biomass accumulation. The addition of 5% CO2 was better than that of 1% CO2 on the biomass and lipid production. The uptakes of nutrients were similar between injection and no injection of CO2, except on phosphorus removal which was affected by the acidification of CO2.





Similar content being viewed by others
References
Lau, P. S., Tam, N. F. Y., & Wong, Y. S. (1996). Wastewater nutrients removal by Chlorella vulgaris: optimization through acclimation. Environmental Technology, 17(2), 183–189.
Hernandez, J. P., de-Bashan, L. E., & Bashan, Y. (2005). Starvation enhances phosphorus removal from wastewater by the microalga Chlorella spp. Co-immobilized with Azospirillum brasilense. Enzyme and Microbial Technology, 38(2006), 190–198.
Li, Y., Chen, Y. F., Chen, P., Min, M., Zhou, W., Martinez, B., et al. (2011). Characterization of a microalgae Chlorella sp. well adapted to highly concentrated municipal wastewater in nutrient removal and biodiesel production. Bioresource Technology, 102(8), 5138–5144.
Wang, L., Wang, Y., Chen, P., & Ruan, R. (2010). Semi-continuous cultivation of Chlorella vulgaris for treating undigested and digested dairy manure. Applied Biochemistry and Biotechnology, 162(8), 2324–2332.
Mulbry, W. W., & Wilkie, A. C. (2001). Growth of benthic freshwater algae on dairy manure. Journal of Applied Phycology, 13, 301–306.
Spatharis, S., Danielidis, D. B., & Tsirtsis, G. T. (2007). Recurrent Pseudo-nitzschia calliantha (Bacillariophyceae) and Alexandrium insuetum (Dinophyceae) winter blooms induced by agricultural runoff. Harmful Algae, 6(6), 811–822.
Kumar, A., Ergas, S., Yuan, X., Sahu, A., Zhang, Q., Dewulf, J., et al. (2010). Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends in Biotechnology, 28, 371–380.
Andrade, M. R., & Costa, J. A. V. (2007). Mixotrophic cultivation of microalga Spirulina platensis using molasses as organic substrate. Aquaculture, 264(1–4), 130–134.
Lee, H. Y., Lee, S. Y., & Park, B. K. (1989). The estimation of algal yield parameters associated with mixotrophic and photoheterotrophic growth under batch cultivation. Biomass, 18(2), 153–160.
Xu, F., Hu, H., Cong, W., Cai, Z., & Ouyang, F. (2004). Growth characteristics and eicosapentaenoic acid production by Nannochloropsis sp. in mixotrophic conditions. Biotechnology Letters, 26(1), 51–53.
Martínez, F., & OrÚs, M. I. (1991). Interactions between glucose and inorganic carbon metabolism in Chlorella vulgaris strain UAM101. Plant Physiology, 95, 1150–1155.
Kobayashi, M., Kakizono, T., Yamagichi, K., Nishio, N., & Nagai, S. (1992). Growth and astaxanthin formation of Haematococcus pluvialls in heterotrophic and mixotrophic conditions. Journal of Fermentation and Bioengineering, 74(1), 17–20.
Chiu, S., Kao, C., Chen, C., Kuan, T., Ong, S., & Lin, C. (2008). Reduction of CO2 by a high-density culture of Chlorella sp. in semicontinuous photobioreactor. Bioresource Technology, 99(9), 3389–3396.
Yang, Y., & Gao, K. (2003). Effects of CO2 concentrations on the freshwater microalgae Chlamydomonas reinhardtii, Chlorella pyrenoidosa and Scenedesmus obliquus (Chlorophyta). Journal of Applied Phycology, 15, 379–389.
Kodama, M., Ikemoto, H., & Miyachi, S. (1993). A new species of highly CO2-tolerant fast-growing marine microalga suitable for high-density culture. Journal of Marine Biotechnology, 1(1), 21–25.
Ingram, L. O., Calder, J. A., Van Baalen, C., Plucker, F. E., & Parker, P. L. (1973). Role of reduced exogenous organic compounds in the physiology of the blue-green bacteria (algae): photoheterotrophic growth of a “heterotrophic” blue-green bacterium. Journal of Bacteriology, 114(2), 695–700.
Baalan, C. V., Pulich, W. M., & Brandeis, M. G. (1973). Heterotrophic growth of the microalgae. CRC Critical Reviews in Microbiology, 2(2), 229–254.
Darley, W. M., Wimpee, B. B., & Ohlman, C. T. (1981). Heterotrophic and photoheterotrophic utilization of lactate by the diatom, Cylindrotheca fusiformis. British Phycological Journal, 16, 423–428.
Zhou, W., Li, Y., Min, M., Hu, B., Chen, P., & Ruan, R. (2011). Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Bioresource Technology. doi:10.1016/j.biortech.2011.04.038.
Huss, V. A., Ciniglia, C., Cennamo, P., Cozzolino, S., Pinto, G., & Pollio, A. (2002). Phylogenetic relationships and taxonomic position of Chlorella-like isolates from low pH environments (pH < 3.0). BMC Evolutionary Biology, 2, 13.
Rippka, R., Deruelles, J., Waterbury, J., Herdman, M., & Stanier, R. (1979). Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Journal of General Microbiology, 111, 1–61.
APHA, AWWA, WEF. (1995). Standard methods for the examination of water and wastewater (19th ed.). Washington: American Public Health Association.
Hach. Procedure manual. (2008) Hach, Loveland, CO.
Folch, J., Lees, M., & Sloane Stanley, G. H. (1956) A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry. 497–509.
Tredici, M. R., Papuzzo, T., & Tomasell, L. (1986). Outdoor mass culture of Spirulina platensis in sea-water. Applied Microbiology and Biotechnology, 24(1), 47–50.
Chen, F., & Zhang, Y. (1997). High cell density mixotrophic culture of Spirulina platensis on glucose for phycocyanin production using a fed-batch system. Enzyme and Microbial Technology, 20(3), 21–224.
Agusti, S., Duarte, C. M., & Kalff, J. (1987). Algal cell size and the maximum density and biomass of phytoplankton. Limnology and Oceanography, 32(4), 983–986.
James, C. M., Al-Khars, A. M., & Chorbani, P. (1988). pH dependent growth of Chlorella in a continuous culture system. Journal of the World Aquaculture Society, 19, 27–35.
Pitter, P. (1976). Determination of biological degradability of organic substrances. Water Research, 10(3), 231–235.
Amblard, C., Couture, P., & Bourdier, G. (1990). Effects of a pulp and paper mill effluent on the structure and metabolism of perphytic algae in experimental streams. Aquatic Toxicology, 18(3), 137–161.
Morris, I., Yentsch, C. M., & Yentsch, C. S. (1971). Relationship between light carbon dioxide fixation and dark carbon dioxide fixation by marine algae. Limnology and Oceanography, 16(6), 854–858.
OECD: Eutrophication of waters. (1982). Monitoring, assessment and control. Pairs: OECD Publications.
González, L. E., Cañizares, R. O., & Baena, S. (1997). Efficiency of ammonia and phosphorus removal from a colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresource Technology, 60, 259–262.
Zhou, A., Tang, H., & Wang, D. (2005). Phosphorus adsorption on natural sediments: modeling and effects of pH and sediment composition. Water Research, 39(7), 1245–1254.
Boyd, C. E. (1982). Water quality management for pond fish culture. Amsterdam: Elsevier Scientific.
Ferrara, R. A., & Avci, C. B. (1982). Nitrogen dynamics in waste stabilization ponds. Journal of the Water Pollution Control Federation, 54(4), 361–369.
Srinath, E. G., & Loehr, R. C. (1974). Ammonia desorption by diffusion aeration. Journal of the Water Pollution Control Federation, 46(8), 1939–1957.
Meron, A. (1971). Kinetics of algal systems in waste treatment field studies. University of California, Berkeley, FWQA, USDOI, PB 206812.
Huppe, H. C., & Turpin, D. H. (1994). Integration of carbon and nitrogen metabolism in plant and algal cells. Annual Review of Plant Physiology and Plant Molecular Biology, 45, 577–607.
Amory, A. M., Vanlerberghe, G. C., & Turpin, D. H. (1991). Demonstration of both a photosynthetic and nonphotosynthetic CO2 requirement for NH +4 assimilation in the green alga Selenastrum minutum. Plant Physiology, 95, 192–96.
Thacker, A., & Syrctt, P. J. (1972). The assimilation of nitrate and ammonium by Chlamydomonas reinhardtii. The New Phytologist, 71, 423–433.
Christie, W. (2003). Lipid analysis, isolation, separation, identification and structural analysis of lipids (3rd ed.). Dundee: MRS Lipids Analysis Unit, Scottish Crop Research Institute, Invergowrie.
Ogbonna, J. C., Masui, H., & Tanaka, H. (1997). Sequentical heterotrophic/autotrophic cultivation—an efficient method of producing Chlorella biomass for health food and animal feed. Journal of Applied Physics, 9(4), 359–366.
Acknowledgement
The study was supported by grants from the University of Minnesota Initiative for Renewable Energy and the Environment (IREE) and Metropolitan Council Environmental Services (MCES), as well as the Legislative-Citizen Commission on Minnesota Resource (LCCMR). The authors are also grateful to Robert C. Polta and Adam Sealock of Saint Paul MCES Wastewater Treatment Plant for hel** with the sample collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, B., Min, M., Zhou, W. et al. Influence of Exogenous CO2 on Biomass and Lipid Accumulation of Microalgae Auxenochlorella protothecoides Cultivated in Concentrated Municipal Wastewater. Appl Biochem Biotechnol 166, 1661–1673 (2012). https://doi.org/10.1007/s12010-012-9566-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9566-2