Abstract
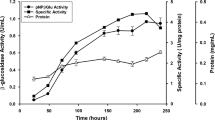
The Pol6 mutant of Penicillium occitanis fungus is of great biotechnological interest since it possesses a high capacity of cellulases and β-glucosidase production with high cellulose degradation efficiency (Jain et al., Enzyme Microb Technol, 12:691–696, 1990; Hadj-Taieb et al., Appl Microbiol Biotechnol, 37:197–201, 1992; Ellouz Chaabouni et al., Enzyme Microb Technol, 16:538–542, 1994; Ellouz Chaabouni et al., Appl Microbiol Biotechnol, 43:267–269, 1995). In this work, two forms of β-glucosidase (β-glu 1 and β-glu 2) were purified from the culture supernatant of the Pol6 strain by gel filtration, ion exchange chromatography, and preparative anionic native electrophoresis. These enzymes were eluted as two distinct species from the diethylamino ethanol Sepharose CL6B and anionic native electrophoresis. However, both behaved identically on sodium dodecyl sulfate polyacrylamide gel electrophoresis (MW, 98 kDa), shared the same amino acid composition, carbohydrate content (8%), and kinetic properties. Moreover, they strongly cross-reacted immunologically. They were active on cellobiose and pNPG with Km values of 1.43 and 0.37 mM, respectively. β-glu 1 and β-glu 2 were competitively inhibited by 1 mM of glucose and 0.03 mM of δ-gluconolactone. They were also significantly inhibited by Hg2+ and Cu2 at 2 mM. The addition of purified enzymes to the poor β-glucosidase crude extract of Trichoderma reesei increased its hydrolytic efficiency on H3P04 swollen cellulose but had no effect with P. occitanis crude extract. Besides their hydrolytic activities, β-glu 1 and β-glu 2 were endowed with trans-glycosidase activity at high concentration of glucose.









Similar content being viewed by others
Abbreviations
- X-glu:
-
5-bromo,4-chloro,3-indolyl,1,4β-glucopyranoside
- Tris:
-
trishydroxymethylaminomethane
- EDTA:
-
ethylene-diaminetetraacetic acid
- p-NP:
-
p-nitrophenol
- pNPG:
-
p-nitrophenyl-β-d-glucopyranoside
- PMSF:
-
phenylmethylsulfonyl fluoride
References
Enari, T. M. (1983). In N. M. Fagarty (Ed.) Microbial cellulases. I. Microbial enzymes and biotechnology pp. 183–223. London: Applied Science Publisher.
Esen, A. (1993). In A. Esen (Ed.) b-glucosidases: Overview, biochemistry and molecular biology pp. 1–14. Washington DC, USA: American Chemical Society.
Bhat, M. K. (2000). Biotechnology Advances, 18, 355–383.
Gargouri, M., Smaali, I., Maugard, T., Legoy, M. D., & Marzouki, N. (2004). Journal of Molecular Catalysis. B, Enzymatic, 29, 89–94.
Jain, S., Parriche, M., Durand, H., & Tiraby, G. (1990). Enzyme and Microbial Technology, 12, 691–696.
Hadj-Taieb, N., Chaabouni Ellouz, S., Kammoun, A., & Ellouz, R. (1992). Applied Microbiology and Biotechnology, 37, 197–201.
Ellouz Chaabouni, S., Hadj-Taieb, N., Mosrati, R., & Ellouz, R. (1994). Enzyme and Microbial Technology, 16, 538–542.
Ellouz Chaabouni, S., Belguith, H., Hsairi, I., M’rad, K., & Ellouz, R. (1995). Applied Microbiology and Biotechnology, 43, 267–269.
Limam, F., Ellouz Chaabouni, S., Ghrir, R., & Marzouki, N. (1995). Enzyme and Microbial Technology, 17, 340–346.
Ellouz Chaabouni, S., Mechichi, T., Limam, F., & Marzouki, N. (2005). Applied Biochemistry and Biotechnology, 125, 99–112.
Mandels, M., & Weber, J. (1969). Advances in Chemistry Series, 95, 391–412.
Wood, T. M., & Bhat, M. K. (1988). In W. A. Wood, & S. T. Kellog (Eds.) Methods in enzymology (vol. 160, pp. 87–116). New York: Academic.
Trinder, J. (1969). Annals of Clinical Biochemistry, 6, 24–25.
Miller, G. L. (1959). Analytical Chemistry, 31, 426–428.
Bradford, M. M. (1976). Analytical Biochemistry, 72, 248–254.
Laemmli, U. K., & Favre, M. (1973). Journal of Molecular Biology, 80, 575–599.
Spiro, R. G. (1966). Methods in Enzymology, 8, 3–26.
Lakshmi, K., Vartack, H. G., & Jagannath, V. (1985). Biotechnology and Bioengineering, 27, 781–785.
Jacksson, M. A., & Dwight, E. T. (1988). Biotechnology and Bioengineering, 23, 903–909.
Kunio, O., Makoto, S., Yasumasa, K., & Shoichi, S. (1985). Journal of Bacteriology, 161, 432–434.
Woodward, J., & Wiseman, A. (1982). Enzyme and Microbial Technology, 4, 73–79.
Chen, H., Hayen, M., & Esterbauer, H. (1992). Biochem Biophys Acta, 1121, 54–60.
McHale, A., & Coughlan, M. P. (1982). Journal of General Microbiology, 128, 2327–2331.
Dekker, R. F. H. (1981). Journal of Microbiology, 127, 177–184.
Hidalgo, M., Steiner, J., & Eyzaguirre, J. (1992). Biotechnology and Applied Biochemistry, 15, 185–191.
Kitpreechavanich, V., Hayachi, M., & Nagai, S. (1986). Agricultural and Biological Chemistry, 50, 1703–1711.
Vanderjagt, D. J., Fry, D. E., & Glew, R. H. (1994). Biochemical Journal, 300, 309–315.
Kuriyama, K. (1995). Bioscience, Biotechnology, and Biochemistry, 59, 1142–1143.
Christakopoulos, P., Goodenough, P. W., Kekos, D., Macris, B. J., Claeyssens, M., & Bhat, M. K. (1994). European Journal of Biochemistry, 224, 379–385.
Watt, D. K., Ono, H., & Hayashi, K. (1998). Biochem Biophys Acta, 1385, 78–88.
Himmel, M. E., Adney, W. S., Fox, J. W., Mitchell, D. J., & Baker, J. O. (1993). Applied Biochemistry and Biotechnology, 39140, 213–225.
Acknowledgements
The authors thank Prof. G. Tiraby and Dr. H. Durand (Cayla Company, France) for kindly supplying the P. occitanis strain used in this work. Prof. R. Ellouz is thanked for his continual support and his interest for the subject.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhiri, F., Chaabouni, S.E., Limam, F. et al. Purification and Biochemical Characterization of Extracellular β-Glucosidases from the Hypercellulolytic Pol6 Mutant of Penicillium occitanis . Appl Biochem Biotechnol 149, 169–182 (2008). https://doi.org/10.1007/s12010-008-8146-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8146-y