Abstract
Background
Endothelin-1 (ET-1) participates in a wide range of cancer-relevant processes including cell proliferation, inhibition of apoptosis, matrix remodeling, bone deposition, and metastases. Although ET-1 reportedly promotes osteosarcoma (OS) cell invasion, suggesting an important role of ET-1 in OS metastasis, the role of ET-1 in OS remains unclear.
Question/purposes
We asked whether (1) ET-1 expression is associated with the malignancy of OS, (2) ET-1 enhances the cell invasion ability of OS, and (3) ET-1 promotes OS cell survival against apoptotic stress.
Methods
We cultured primary OS specimens from 22 patients with Stages II (OS-II) and III (OS-III) in real-time quantitative RT-PCR and ELISA to compare ET-1 expression. We used Transwell® cell invasion assays (in triplicate) to assess the invasion ability of cells in the presence or absence of exogenous ET-1 and/or ET receptor antagonists. We compared cell apoptosis rate among the cells treated with cisplatin in the presence or absence of exogenous ET-1 and/or ET receptor antagonists. We used OS cell line MG-63 in all experiments as a reference.
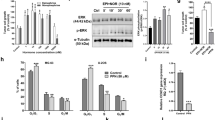
Results
Real-time quantitative RT-PCR and ELISA showed OS-III cells had greater ET-1 expression than OS-II cells at the mRNA and the secreted protein levels. Transwell® cell invasion assays showed OS-III cells had a greater migrated cell number than OS-II cells, which could be abrogated by ETA receptor antagonist BQ123 (100 pmol/L), but not ETB receptor antagonist BQ788 (1 μmol/L); exogenous ET-1 dose-dependently promoted OS cell migration, which could be inhibited by BQ123 (100 pmol/L). Cisplatin (10 nmol/L) induced less apoptosis in OS-III cells than in OS-II cells; exogenous ET-1 dose-dependently promoted OS cell survival against cisplatin-induced apoptosis; both effects were reversed by BQ123 (1 μmol/L), but not BQ788 (1 μmol/L).
Conclusions
Increased ET-1 expression appears to be associated with increased malignancy of OS. ET-1 promotes OS cell invasion and survival against cisplatin-induced apoptosis through the ETA receptor.
Clinical Relevance
The ET-1/ETA pathway may represent an important target for treating OS, because blocking the ETA receptor with a selective antagonist can inhibit OS cell invasion and potentiate a chemotherapeutic agent’s effect on OS.





Similar content being viewed by others
References
Baba AI, Catoi C. Tumor Cell Morphology. In: Baba AI, Catoi C, eds. Comparative Oncology. Bucharest, Romania: The Publishing House of the Romanian Academy, 2007:119–125.
Bacci G, Briccoli A, Rocca M, Ferrari S, Donati D, Longhi A, Bertoni F, Bacchini P, Giacomini S, Forni C, Manfrini M, Galletti S. Neoadjuvant chemotherapy for OS of the extremities with metastases at presentation: recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann Oncol. 2003;14:1126–1134.
Bagnato A, Salani D, Di Castro V. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res. 1999;59:720–727.
Benjamin RS, Patel SR. Pediatric and adult osteosarcoma: comparison and contrasts in presentation and therapy. Cancer Treat Res. 2010;152: 355–363.
Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120.
Felx M, Guyot MC, Isler M, Turcotte RE, Doyon J, Khatib AM, Leclerc S, Moreau A, Moldovan F. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-kappaB in human osteosarcoma. Clin Sci (Lond). 2006;110:645–654.
Giaid A, Hamid QA, Springall DR, Yanagisawa M, Shinmi O, Sawamura T, Masaki T, Kimura S, Corrin B, Polak JM. Detection of endothelin immunoreactivity and mRNA in pulmonary tumours. J Pathol. 1990;162:15–22.
Gorlick R, Anderson P, Andrulis I, Arndt C, Beardsley GP, Bernstein M, Bridge J, Cheung NK, Dome JS, Ebb D, Gardner T, Gebhardt M, Grier H, Hansen M, Healey J, Helman L, Hock J, Houghton J, Houghton P, Huvos A, Khanna C, Kieran M, Kleinerman E, Ladanyi M, Lau C, Malkin D, Marina N, Meltzer P, Meyers P, Schofield D, Schwartz C, Smith MA, Toretsky J, Tsokos M, Wexler L, Wigginton J, Withrow S, Schoenfeldt M, Anderson B. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary. Clin Cancer Res. 2003;9:5442–5453.
Graat HC, Witlox MA, Schagen FH, Kaspers GJ, Helder MN, Bras J, Schaap GR, Gerritsen WR, Wuisman PI, van Beusechem VW. Different susceptibility of osteosarcoma cell lines and primary cells to treatment with oncolytic adenovirus and doxorubicin or cisplatin. Br J Cancer. 2006;94:1837–1844.
Hopkin K, Edwards P, Harris A, Klausner R, Peters G, Selby P, Stanley M. Cancer. In: Alberts B, Johnson A, Lewis J, eds. Molecular Biology of the Cell. 4th ed. New York, NY: Garland Science; 2002:1324–1325.
Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC, Lu KH. Silibinin suppresses human osteosarcoma MG-63 cell invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of MMP-2. Carcinogenesis. 2007;28:977–987.
Kager L, Zoubek A, Potschger U, Kastner U, Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M, Winkelmann W, Jundt G, Kabisch H, Reichardt P, Jürgens H, Gadner H, Bielack SS. Primary metastatic OS: presentation and outcome of patients treated on neoadjuvant Cooperative OS Study Group protocols. J Clin Oncol. 2003;21: 2011–2018.
Kawasaki M, Maeda T, Hanasawa K, Ohkubo I, Tani T. Effect of His-Gly-Lys motif derived from domain 5 of high molecular weight kininogen on suppression of cancer metastasis both in vitro and in vivo. J Biol Chem. 2003;278:49301–49307.
Kurihara M, Ochi A, Kawaguchi T, Niwa M, Kataoka Y, Mori K. Localization and characterization of endothelin receptors in human gliomas: a growth factor? Neurosurgery.1990;27:275–281.
Lahn M, Köhler G, Schmoor C, Dengler W, Veelken H, Brennscheidt U, Mackensen A, Kulmburg P, Hentrich I, Jesuiter H, Rosenthal FM, Fiebig HH, Sommerkamp H, Farthmann EH, Hasse J, Mertelsmann R, Lindemann A. Processing of tumor tissues for vaccination with autologous tumor cells. Eur Surg Res. 1997;29:292–302.
Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival–a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–638.
Nakamuta M, Ohashi M, Tabata S, Tanabe Y, Goto K, Naruse M, Naruse K, Hiroshige K, Nawata H. High plasma concentrations of endothelin-like immunoreactivities in patients with hepatocellular carcinoma. Am J Gastroenterol. 1993;88:248–252.
Nelson J, Bagnato A, Battistini B, and Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer 2003;3:110–116.
Nelson JB, Hedican SP, George DJ. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995;1:944–949.
Nelson JB, Nguyen SH, Wu-Wong JR, Opgenorth TJ, Dixon BD, Chung LW, Inoue N. New bone formation in an osteoblastic tumour model is increased by endothelin-1 overexpression and decreased by ETA receptor blockade. Urology. 1999;53:1063–1069.
Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2010;152:3–13.
Pinkas J, Martin SS, Leder P. Bcl-2-mediated cell survival promotes metastasis of EpH4 βMEKDD mammary epithelial cells. Mol Cancer Res. 2004;2:551–556.
Reddy RM, Tsai WS, Ziauddin MF, Zuo J, Cole GW Jr, Maxhimer JB, Fang B, Schrump DS, Nguyen DM. Cisplatin enhances apoptosis induced by a tumor-selective adenovirus expressing tumor necrosis factor-related apoptosis-inducing ligand. J Thorac Cardiovasc Surg. 2004;128:883–891.
Rosano L, Varmi M, Salani D, Di CV, Spinella F, Natali PG, Bagnato A. Endothelin-1 induces tumour proteinase activation and invasiveness of ovarian carcinoma cells. Cancer Res. 2001;61:8340–8346.
Rosenberg B, Vancamp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222:385–386.
Schwerdt G, Freudinger R, Schuster C, Weber F, Thews O, Gekle M. Cisplatin-induced apoptosis is enhanced by hypoxia and by inhibition of mitochondria in renal collecting duct cells. Toxicol Sci. 2005;85:735–742.
Schweyer S, Soruri A, Meschter O, Heintze A, Zschunke F, Miosge N, Thelen P, Schlott T, Radzun HJ, Fayyazi A. Cisplatin-induced apoptosis in human malignant testicular germ cell lines depends on MEK/ERK activation. Br J Cancer. 2004;91:589–598.
Shankar A, Loizidou M, Aliev G, Fredericks S, Holt D, Boulos PB, Burnstock G, Taylor I. Raised endothelin 1 levels in patients with colorectal liver metastases. Br J Surg. 1998;85:502–506.
Shi WX, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, leask A, Abraham DJ. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719.
Shichiri M, Hirata Y, Nakajima T, Ando K, Imai T, Yanagisawa M, Masaki T, Marumo F. Endothelin-1 is an autocrine/paracrine growth factor for human cancer cell lines. J Clin Invest. 1991;87:1867–1871.
Tastesen HS, Holm JB, Møller J, Poulsen KA, Møller C, Stürup S, Hoffmann EK, Lambert IH. Pinpointing differences in cisplatin-induced apoptosis in adherent and non-adherent cancer cells. Cell Physiol Biochem. 2010;26:809–820.
Wada T, Isu K, Takeda N, Usui M, Ishii S, Yamawaki S. A preliminary report of neoadjuvant chemotherapy NSH-7 study in osteosarcoma: preoperative salvage chemotherapy based on clinical tumor response and the use of granulocyte colony-stimulating factor. Oncology 1996;53:221–227.
**e X, Chen JW, Li F, Tian J, Gao JS, Zhang D. A T-cell-based enzyme-linked immunospot assay for tuberculosis screening in Chinese patients with rheumatic diseases receiving infliximab therapy. Clin Exp Med. 2010; DOI: 10.1007/s10238-010-0123-4.
Yu L, Wang CY, Miao L, Du X, Mayer D, Zhang J. Estrogens promote invasion of prostate cancer cells in a paracrine manner through up-regulation of matrix metalloproteinase 2 in prostatic stromal cells. Endocrinol. 2011;152:773–781.
Acknowledgments
We thank Drs. Hong Yu and Lin Chen (Department of Pathology, **angya School of Medicine, Changsha, China) for technical support on identification of tumor cells in primary osteosarcoma cell cultures. We thank Prof. Bin Shan (Department of Medicine, Tulane University, LA, USA) for help with preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Zhao, Y., Liao, Q., Zhu, Y. et al. Endothelin-1 Promotes Osteosarcoma Cell Invasion and Survival against Cisplatin-induced Apoptosis. Clin Orthop Relat Res 469, 3190–3199 (2011). https://doi.org/10.1007/s11999-011-1939-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-011-1939-2