Abstract
Purpose of Review
This review provides an overview of the potential pathogenic roles of anti-SRP and anti-HMGCR in IMNM over the past 5 years.
Recent Findings
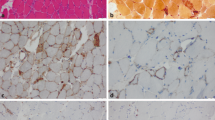
Idiopathic inflammatory myopathies (IIM) are a group of acquired autoimmune disorders that mainly affect the skeletal muscle tissue. Classification criteria of IIM are comprised of polymyositis, dermatomyositis, inclusion body myositis and immune-mediated necrotizing myopathies. One important hallmark of autoimmune diseases is the detection of autoantibodies in patient sera. The anti-SRP (signal recognition particle) and anti-HMGCR (3-hydroxy-3-methylglutaryl coenzyme A reductase) antibodies are specifically associated with IMNM patients, and their detection has been described as related to disease severity. The muscles of IMNM patients are characterized by necrosis, atrophy and regenerating fibres with sparse inflammatory infiltrates.
Summary
Although an important correlation between autoantibody titres, creatine kinase levels and disease progression/severity has been described in the last few years, the potential pathogenic roles of these autoantibodies have only recently been described.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dalakas MC. Inflammatory Muscle Diseases. N Engl J Med. 2015;373(4):393–4.
Hoogendijk JE, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscul Disord. 2004;14(5):337–45.
Mammen AL, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63(3):713–21.
Allenbach Y, et al. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine (Baltimore). 2014;93(3):150–7.
Hengstman GJ, et al. Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann Rheum Dis. 2006;65(12):1635–8.
• Watanabe Y, et al. Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy. J Neurol Neurosurg Psychiatry. 2016;87(10):1038–44. This study provides a thorough clinical description of anti-SRP and anti-HMGCR in IMNM
Musset L, et al. Anti-HMGCR antibodies as a biomarker for immune-mediated necrotizing myopathies: A history of statins and experience from a large international multi-center study. Autoimmun Rev. 2016;15(10):983–93.
Suzuki S, et al. Integrated Diagnosis Project for Inflammatory Myopathies: An association between autoantibodies and muscle pathology. Autoimmun Rev. 2017;16(7):693–700.
Christopher-Stine L, et al. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62(9):2757–66.
• Benveniste O, Stenzel W, Allenbach Y. Advances in serological diagnostics of inflammatory myopathies. Curr Opin Neurol. 2016;29(5):662–73. This study provides a thorough description of autoantibodies in IMM
Brouwer R, et al. Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis. 2001;60(2):116–23.
• Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med. 2016;280(1):8–23. This study provides a thorough description of autoantibodies in IMM
Reeves WH, Nigam SK, Blobel G. Human autoantibodies reactive with the signal-recognition particle. Proc Natl Acad Sci U S A. 1986;83(24):9507–11.
Christopher-Stine L. Neurologists are from Mars. Rheumatologists are from Venus: differences in approach to classifying the idiopathic inflammatory myopathies. Curr Opin Rheumatol. 2010;22(6):623–6.
Drouot L, et al. Exploring necrotizing autoimmune myopathies with a novel immunoassay for anti-3-hydroxy-3-methyl-glutaryl-CoA reductase autoantibodies. Arthritis Res Ther. 2014;16(1):R39.
Mammen AL. Statin-Associated Autoimmune Myopathy. N Engl J Med. 2016;374(7):664–9.
Allenbach Y, et al. High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain. 2016;139(Pt 8):2131–5.
• Pinal-Fernandez I, et al. Thigh muscle MRI in immune-mediated necrotising myopathy: extensive oedema, early muscle damage and role of anti-SRP autoantibodies as a marker of severity. Ann Rheum Dis. 2017;76(4):681–7. This study provides a thorough clinical description of anti-SRP severity in IMNM
Tiniakou E, et al. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology (Oxford). 2017;56(5):787–94.
Pinal-Fernandez I, et al. Longitudinal Course of Disease in a Large Cohort of Myositis Patients With Autoantibodies Recognizing the Signal Recognition Particle. Arthritis Care Res (Hoboken). 2017;69(2):263–70.
Mammen AL, Tiniakou E. Intravenous Immune Globulin for Statin-Triggered Autoimmune Myopathy. N Engl J Med. 2015;373(17):1680–2.
Bergua C, et al. Immune-mediated necrotizing myopathy. Z Rheumatol. 2016;75(2):151–6.
Suzuki S, et al. Inflammatory myopathy with anti-signal recognition particle antibodies: case series of 100 patients. Orphanet J Rare Dis. 2015;10:61.
Valiyil R, et al. Rituximab therapy for myopathy associated with anti-signal recognition particle antibodies: a case series. Arthritis Care Res (Hoboken). 2010;62(9):1328–34.
•• Pinal-Fernandez I, Mammen AL. Spectrum of immune-mediated necrotizing myopathies and their treatments. Curr Opin Rheumatol. 2016;28(6):619–24. This study provides a thorough clinical description of IMNM
Walter P, Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980;77(12):7112–6.
Walter P, Blobel G. Disassembly and reconstitution of signal recognition particle. Cell. 1983;34(2):525–33.
Walter P, Gilmore R, Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984;38(1):5–8.
Keenan RJ, et al. The signal recognition particle. Annu Rev Biochem. 2001;70:755–75.
Luirink J, Sinning I. SRP-mediated protein targeting: structure and function revisited. Biochim Biophys Acta. 2004;1694(1-3):17–35.
Saraogi I, Shan SO. Molecular mechanism of co-translational protein targeting by the signal recognition particle. Traffic. 2011;12(5):535–42.
Römisch K, et al. Human autoantibodies against the 54 kDa protein of the signal recognition particle block function at multiple stages. Arthritis Res Ther. 2006;8(2):R39.
Benveniste O, et al. Correlation of anti-signal recognition particle autoantibody levels with creatine kinase activity in patients with necrotizing myopathy. Arthritis Rheum. 2011;63(7):1961–71.
Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–30.
Istvan ES, et al. Crystal structure of the catalytic portion of human HMG-CoA reductase: insights into regulation of activity and catalysis. EMBO J. 2000;19(5):819–30.
Roitelman J, et al. Immunological evidence for eight spans in the membrane domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase: implications for enzyme degradation in the endoplasmic reticulum. J Cell Biol. 1992;117(5):959–73.
Hamann PD, et al. Statin-induced necrotizing myositis - a discrete autoimmune entity within the "statin-induced myopathy spectrum". Autoimmun Rev. 2013;12(12):1177–81.
Alshehri A, et al. Myopathy with anti-HMGCR antibodies: Perimysium and myofiber pathology. Neurol Neuroimmunol Neuroinflamm. 2015;2(4):e124.
Werner JL, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum. 2012;64(12):4087–93.
Arlet JB, et al. Marked efficacy of a therapeutic strategy associating prednisone and plasma exchange followed by rituximab in two patients with refractory myopathy associated with antibodies to the signal recognition particle (SRP). Neuromuscul Disord. 2006;16(5):334–6.
•• Allenbach Y, et al. Necrosis in anti-SRP. Neurology. 2018;90(6):e507–17. This study provides a thorough description of the anti-SRP and anti-HMGCR pathogenic role
De Bleecker JL, et al. 205th ENMC International Workshop: Pathology diagnosis of idiopathic inflammatory myopathies part II 28-30 March 2014, Naarden, The Netherlands. Neuromuscul Disord. 2015;25(3):268–72.
Miller T, et al. Myopathy with antibodies to the signal recognition particle: clinical and pathological features. J Neurol Neurosurg Psychiatry. 2002;73(4):420–8.
Dimitri D, et al. Myopathy associated with anti-signal recognition peptide antibodies: clinical heterogeneity contrasts with stereotyped histopathology. Muscle Nerve. 2007;35(3):389–95.
Stenzel W, Goebel HH, Aronica E. Review: immune-mediated necrotizing myopathies--a heterogeneous group of diseases with specific myopathological features. Neuropathol Appl Neurobiol. 2012;38(7):632–46.
Chung T, et al. The composition of cellular infiltrates in anti-HMG-CoA reductase-associated myopathy. Muscle Nerve. 2015;52(2):189–95.
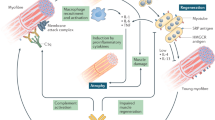
Bencze M, et al. Proinflammatory macrophages enhance the regenerative capacity of human myoblasts by modifying their kinetics of proliferation and differentiation. Mol Ther. 2012;20(11):2168–79.
Saclier M, et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31(2):384–96.
Rojana-udomsart A, et al. Complement-mediated muscle cell lysis: a possible mechanism of myonecrosis in anti-SRP associated necrotizing myopathy (ASANM). J Neuroimmunol. 2013;264(1-2):65–70.
•• Arouche-Delaperche, L., et al., Pathogenic role of anti-SRP and anti-HMGCR antibodies in necrotizing myopathies: Myofiber atrophy and impairment of muscle regeneration in necrotizing autoimmune myopathies. Ann Neurol, 2017. This study provides a thorough description of the anti-SRP and anti-HMGCR pathogenic role.
Trapani L, et al. 3-hydroxy 3-methylglutaryl coenzyme A reductase inhibition impairs muscle regeneration. J Cell Biochem. 2012;113(6):2057–63.
McNally AK, Anderson JM. Multinucleated giant cell formation exhibits features of phagocytosis with participation of the endoplasmic reticulum. Exp Mol Pathol. 2005;79(2):126–35.
Okazaki Y, et al. Cell surface expression of calnexin, a molecular chaperone in the endoplasmic reticulum. J Biol Chem. 2000;275(46):35751–8.
Mammen AL, et al. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res (Hoboken). 2012;64(8):1233–7.
Ohnuki Y, et al. HLA-DRB1 alleles in immune-mediated necrotizing myopathy. Neurology. 2016;87(18):1954–5.
Yanase K, et al. Receptor-mediated cellular entry of nuclear localizing anti-DNA antibodies via myosin 1. J Clin Invest. 1997;100(1):25–31.
Yanase K, Madaio MP. Nuclear localizing anti-DNA antibodies enter cells via caveoli and modulate expression of caveolin and p53. J Autoimmun. 2005;24(2):145–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Inflammatory Muscle Disease
Rights and permissions
About this article
Cite this article
Ladislau, L., Arouche-Delaperche, L., Allenbach, Y. et al. Potential Pathogenic Role of Anti-Signal Recognition Protein and Anti-3-hydroxy-3-methylglutaryl-CoA Reductase Antibodies in Immune-Mediated Necrotizing Myopathies. Curr Rheumatol Rep 20, 56 (2018). https://doi.org/10.1007/s11926-018-0763-z
Published:
DOI: https://doi.org/10.1007/s11926-018-0763-z