Abstract
Purpose of Review
There is a growing appreciation within the scientific community that cells exhibit regional variation. Whether the variation is attributable to differences in embryonic origin or anatomical location and mechanical loading has not been elucidated; what is clear, however, is that adult cells carry positional information that ultimately affects their functions. The purpose of this review is to highlight the functions of osteocytes in the craniomaxillofacial (CMF) skeleton as opposed to elsewhere in the body, and in doing so gain mechanistic insights into genetic conditions and chemically-induced diseases that particularly affect this region of our anatomy.
Recent Findings.
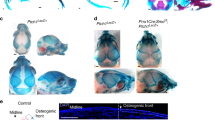
In the CMF skeleton, elevated Wnt/β-catenin signaling affects not only bone mass and volume, but also mineralization of the canalicular network and osteocyte lacunae. Aberrant elevation in the Wnt/β-catenin pathway can also produce micropetrosis and osteonecrosis of CMF bone, presumably due to a disruption in the signaling network that connects osteocytes to one another, and to osteoblasts on the bone surface.





Similar content being viewed by others
Data Availability
The data presented in this study are available upon request from the corresponding author.
References
Goldring, S. R. The osteocyte: key player in regulating bone turnover. RMD Open 2015; 1: e000049 2015. https://doi.org/10.1136/rmdopen-2015-000049.
Heino TJ, Kurata K, Higaki H, Vaananen HK. Evidence for the role of osteocytes in the initiation of targeted remodeling. Technol Health Care. 2009;17:49–56. https://doi.org/10.3233/THC-2009-0534.
Kelder C, Kleverlaan C J, Gilijamse M, Bakker A D & de Vries T J Cells derived from human long bone appear more differentiated and more actively stimulate osteoclastogenesis compared to alveolar bone-derived cells. Int J Mol Sci 2020;21. https://doi.org/10.3390/ijms21145072.
Wan Q, et al. Osteoblasts of calvaria induce higher numbers of osteoclasts than osteoblasts from long bone. Bone. 2016;86:10–21. https://doi.org/10.1016/j.bone.2016.02.010.
Cane V, et al. Size and density of osteocyte lacunae in different regions of long bones. Calcif Tissue Int. 1982;34:558–63. https://doi.org/10.1007/BF02411304.
Almasoud NN, Tanneru N, Marei HF. Alveolar bone density and its clinical implication in the placement of dental implants and orthodontic mini-implants. Saudi Med J. 2016;37:684–9. https://doi.org/10.15537/Smj.2016.6.14274.
Chen CH, et al. An osteopenic/osteoporotic phenotype delays alveolar bone repair. Bone. 2018;112:212–9. https://doi.org/10.1016/j.bone.2018.04.019.
Kuroshima S, Al-Omari FA, Sasaki M, Sawase T. Medication-related osteonecrosis of the jaw: a literature review and update. Genesis. 2022;60:e23500. https://doi.org/10.1002/dvg.23500.
Gardner JC, et al. Bone mineral density in sclerosteosis; affected individuals and gene carriers. J Clin Endocrinol Metab. 2005;90:6392–5. https://doi.org/10.1210/jc.2005-1235.
van Bezooijen RL, et al. Sclerostin in mineralized matrices and van Buchem disease. J Dent Res. 2009;88:569–74. https://doi.org/10.1177/0022034509338340.
Harris SE, et al. Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects. Bone. 2012;50:42–53. https://doi.org/10.1016/j.bone.2011.09.038.
Kirkpatrick DB, Rimoin DL, Kaitila I, Goodman SJ. The craniotubular bone modeling disorders: a neurosurgical introduction to rare skeletal dysplasias with cranial nerve compression. Surg Neurol. 1977;7:221–32.
Wolff J, in The law of bone remodeling (ed Hirschwald) (Springer, 1892).
Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. https://doi.org/10.1002/ar.1092190104.
Frost HM. From Wolff’s law to the Utah paradigm: insights about bone physiology and its clinical applications. Anat Rec. 2001;262:398–419.
Organ C, Nunn CL, Machanda Z, Wrangham RW. Phylogenetic rate shifts in feeding time during the evolution of Homo. Proc Natl Acad Sci U S A. 2011;108:14555–9. https://doi.org/10.1073/pnas.1107806108.
Brachetta-Aporta N, Gonzalez PN, Bernal V. Variation in facial bone growth remodeling in prehistoric populations from southern South America. Am J Phys Anthropol. 2019;169:422–34. https://doi.org/10.1002/ajpa.23857.
Allen M, R & Burr D, B The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 2009; 67: 61-70. https://doi.org/10.1016/j.joms.2009.01.007.
Burr DB, Allen MR. Mandibular necrosis in beagle dogs treated with bisphosphonates. Orthod Craniofac Res. 2009;12:221–8. https://doi.org/10.1111/j.1601-6343.2009.01456.x.
Huja S S, & Beck F, M Bone remodeling in maxilla, mandible, and femur of young dogs. Anatomical record (Hoboken, N.J. 2007) 2008; 291: 1–5. https://doi.org/10.1002/ar.20619.
Utreja A, Motevasel H, Bain C, Holland R, Robling A. The effect of overexpression of Lrp5 on the temporomandibular joint. Cartilage. 2021;13:419S-426S. https://doi.org/10.1177/1947603520968875.
Kondo T, Wakabayashi N. Influence of molar support loss on stress and strain in premolar periodontium: a patient-specific FEM study. J Dent. 2009;37:541–8. https://doi.org/10.1016/j.jdent.2009.03.015.
Xu Q, et al. Mechanoadaptive responses in the periodontium are coordinated by Wnt. J Dent Res. 2019;98:689–97. https://doi.org/10.1177/0022034519839438.
Zhang X, et al. Molecular basis for periodontal ligament adaptation to in vivo loading. J Dent Res. 2019;98:331–8. https://doi.org/10.1177/0022034518817305.
Inoue M, et al. Forceful mastication activates osteocytes and builds a stout jawbone. Sci Rep. 2019;9:4404. https://doi.org/10.1038/s41598-019-40463-3.
Kingsmill VJ, Boyde A, Davis GR, Howell PG, Rawlinson SC. Changes in bone mineral and matrix in response to a soft diet. J Dent Res. 2010;89:510–4. https://doi.org/10.1177/0022034510362970.
Kawakami T, Takise S, Fuchimoto T, Kawata H. Effects of masticatory movement on cranial bone mass and micromorphology of osteocytes and osteoblasts in develo** rats. Asia Pac J Clin Nutr. 2009;18:96–104.
Robling AG, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75. https://doi.org/10.1074/jbc.M705092200.
Zhao D, et al. Osteocytes regulate bone anabolic response to mechanical loading in male mice via activation of integrin alpha5. Bone Res. 2022;10:49. https://doi.org/10.1038/s41413-022-00222-z.
Fulzele K, et al. Loss of Gsalpha in osteocytes leads to osteopenia due to sclerostin induced suppression of osteoblast activity. Bone. 2018;117:138–48. https://doi.org/10.1016/j.bone.2018.09.021.
Robinson D, et al. Load response of the natural tooth and dental implant: a comparative biomechanics study. J Adv Prosthodont. 2019;11:169–78. https://doi.org/10.4047/jap.2019.11.3.169.
Tian Y, Sadowsky S J, Brunski J B, Yuan X & Helms J, A Effects of masticatory loading on bone remodeling around teeth vs. implants: insights from a preclinical model. Clin Oral Implants Res 2022. https://doi.org/10.1111/clr.13894.
Okawara H, et al. Effect of load-induced local mechanical strain on peri-implant bone cell activity related to bone resorption and formation in mice: an analysis of histology and strain distributions. J Mech Behav Biomed Mater. 2021;116:104370. https://doi.org/10.1016/j.jmbbm.2021.104370.
Mouraret S, et al. A pre-clinical murine model of oral implant osseointegration. Bone. 2014;58:177–84. https://doi.org/10.1016/j.bone.2013.07.021.
Coyac BR, et al. A novel system exploits bone debris for implant osseointegration. J Periodontol. 2021;92:716–26. https://doi.org/10.1002/JPER.20-0099.
Robling AG, Bonewald LF. The osteocyte: new insights. Annu Rev Physiol. 2020;82:485–506. https://doi.org/10.1146/annurev-physiol-021119-034332.
Rochefort GY, Pallu S, Benhamou CL. Osteocyte: the unrecognized side of bone tissue. Osteoporos Int. 2010;21:1457–69. https://doi.org/10.1007/s00198-010-1194-5.
Schurman C A, Verbruggen S W, & Alliston T, Disrupted osteocyte connectivity and pericellular fluid flow in bone with aging and defective TGF-beta signaling. Proc Natl Acad Sci U S A 2021;118. https://doi.org/10.1073/pnas.2023999118.
Moriishi T, et al. Sp7 Transgenic mice with a markedly impaired lacunocanalicular network induced sost and reduced bone mass by unloading. Int J Mol Sci 23:2022. https://doi.org/10.3390/ijms23063173
Tamplen M, et al. Treatment with anti-sclerostin antibody to stimulate mandibular bone formation. Head Neck. 2018;40:1453–60. https://doi.org/10.1002/hed.25128.
Schwarze UY, Dobsak T, Gruber R, Bookstein FL. Anatomical similarity between the Sost-knockout mouse and sclerosteosis in humans. Anat Rec (Hoboken). 2020;303:2295–308. https://doi.org/10.1002/ar.24318.
Chen J, et al. Molecular basis for craniofacial phenotypes caused by sclerostin deletion. J Dent Res. 2021;100:310–7. https://doi.org/10.1177/0022034520963584.
Stein SA, et al. Sclerosteosis: neurogenetic and pathophysiologic analysis of an American kinship. Neurology. 1983;33:267–77. https://doi.org/10.1212/wnl.33.3.267.
O’Brien CA, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One. 2008;3:e2942. https://doi.org/10.1371/journal.pone.0002942.
Vezeridis PS, Semeins CM, Chen Q, Klein-Nulend J. Osteocytes subjected to pulsating fluid flow regulate osteoblast proliferation and differentiation. Biochem Biophys Res Commun. 2006;348:1082–8. https://doi.org/10.1016/j.bbrc.2006.07.146.
Koide M, et al. Evidence for the major contribution of remodeling-based bone formation in sclerostin-deficient mice. Bone. 2022;160:116401. https://doi.org/10.1016/j.bone.2022.116401.
Donham C, et al Sclerostin depletion induces inflammation in the bone marrow of mice. Int J Mol Sci 2021;22. https://doi.org/10.3390/ijms22179111.
Moustafa A, et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23:1225–34. https://doi.org/10.1007/s00198-011-1656-4.
Niziolek PJ, et al. High-bone-mass-producing mutations in the Wnt signaling pathway result in distinct skeletal phenotypes. Bone. 2011;49:1010–9. https://doi.org/10.1016/j.bone.2011.07.034.
Li X, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–7. https://doi.org/10.1074/jbc.M413274200.
Chen J, et al Wnt/beta-catenin signaling controls maxillofacial hyperostosis. J Dent Res, 2022; 220345211067705. https://doi.org/10.1177/00220345211067705.
Tu X, et al. Osteocytes mediate the anabolic actions of canonical Wnt/beta-catenin signaling in bone. Proc Natl Acad Sci U S A. 2015;112:E478-486. https://doi.org/10.1073/pnas.1409857112.
Du JH, et al. The function of Wnt ligands on osteocyte and bone remodeling. J Dent Res. 2019;98:930–8. https://doi.org/10.1177/0022034519854704.
Lim WH, et al. Wnt signaling regulates homeostasis of the periodontal ligament. J Periodontal Res. 2014;49:751–9. https://doi.org/10.1111/jre.12158.
Lim WH, Liu B, Mah SJ, Yin X, Helms JA. Alveolar bone turnover and periodontal ligament width are controlled by Wnt. J Periodontol. 2015;86:319–26. https://doi.org/10.1902/jop.2014.140286.
Frost H, M Micropetrosis, J Bone Joint Surg Am 1960; 42-A: 144–150.
Milovanovic P, & Busse B, Phenomenon of osteocyte lacunar mineralization: indicator of former osteocyte death and a novel marker of impaired bone quality? Endocr Connect 2020. https://doi.org/10.1530/EC-19-0531.
Milovanovic P, Busse B. Phenomenon of osteocyte lacunar mineralization: indicator of former osteocyte death and a novel marker of impaired bone quality? Endocr Connect. 2020;9:R70–80. https://doi.org/10.1530/EC-19-0531.
McKenzie J, et al. Osteocyte death and bone overgrowth in mice lacking fibroblast growth factor receptors 1 and 2 in mature osteoblasts and osteocytes. J Bone Miner Res. 2019;34:1660–75. https://doi.org/10.1002/jbmr.3742.
Milovanovic P, et al The Formation of calcified nanospherites during micropetrosis represents a unique mineralization mechanism in aged human bone. Small 2017 13. https://doi.org/10.1002/smll.201602215.
Vashishth D, Gibson G, Kimura J, Schaffler MB, Fyhrie DP. Determination of bone volume by osteocyte population. Anat Rec. 2002;267:292–5. https://doi.org/10.1002/ar.10114.
Schemenz V, et al. Heterogeneity of the osteocyte lacuno-canalicular network architecture and material characteristics across different tissue types in healing bone. J Struct Biol. 2020;212:107616. https://doi.org/10.1016/j.jsb.2020.107616.
Reid IR, Cundy T. Osteonecrosis of the jaw. Skeletal Radiol. 2009;38:5–9. https://doi.org/10.1007/s00256-008-0549-x.
Reichenberger, E. & Chen, I.-P. Craniometaphyseal dysplasia, autosomal dominant, <https://www.ncbi.nlm.nih.gov/books/NBK1461/. > (2007).
Chen, I. P., Wang, L., Jiang, X., Aguila, H. L. & Reichenberger, E. J. A Phe377del mutation in ANK leads to impaired osteoblastogenesis and osteoclastogenesis in a mouse model for craniometaphyseal dysplasia (CMD). Hum Mol Genet 2011;20: 948-961. https://doi.org/10.1093/hmg/ddq541.
Hu Y, et al. A novel autosomal recessive GJA1 missense mutation linked to Craniometaphyseal dysplasia. PLoS One. 2013;8:e73576. https://doi.org/10.1371/journal.pone.0073576.
Hayashibara T, Komura T, Sobue S, Ooshima T. Tooth eruption in a patient with craniometaphyseal dysplasia: case report. J Oral Pathol Med. 2000;29:460–2. https://doi.org/10.1034/j.1600-0714.2000.290907.x.
Wu Y, et al. Aberrantly elevated Wnt signaling is responsible for cementum overgrowth and dental ankylosis. Bone. 2019;122:176–83. https://doi.org/10.1016/j.bone.2018.10.023.
Gorlin R J, Cohen M M & Levin L S, Syndromes of the head and neck. 3rd edn, Vol. 1 (Oxford University Press, 1990).
Zhou Y, et al. Aberrant activation of Wnt signaling pathway altered osteocyte mineralization. Bone. 2019;127:324–33. https://doi.org/10.1016/j.bone.2019.06.027.
Soares M Q S. et al. High doses of zoledronic acid induce differential effects on femur and jawbone microstructure. Clin Exp Dent Res 2022; https://doi.org/10.1002/cre2.643.
Adamo V, et al. Current knowledge and future directions on bisphosphonate-related osteonecrosis of the jaw in cancer patients. Expert Opin Pharmacother. 2008;9:1351–61. https://doi.org/10.1517/14656566.9.8.1351.
Silverman SL, &. Landesberg, R. Osteonecrosis of the jaw and the role of bisphosphonates: a critical review. Am J Med. 2009;122:S33-45. https://doi.org/10.1016/j.amjmed.2008.12.005.
Wessel J H, Dodson T B, & Zavras A I, Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: a case-control study. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 2008;66: 625-631. https://doi.org/10.1016/j.joms.2007.11.032.
Griffin A, Brain P, Hancock C, Jeyapalina SA. Dentist’s perspective on the need for interdisciplinary collaboration to reduce medication-related osteonecrosis of the jaw. Sr Care Pharm. 2022;37:458–67. https://doi.org/10.4140/TCP.n.2022.458.
Shimizu, R. et al. Incidence and risk of anti-resorptive agent-related osteonecrosis of the jaw after tooth extraction: a retrospective study. Healthcare (Basel) 2022;10. https://doi.org/10.3390/healthcare10071332.
Jiang C, et al. Development and validation of a prediction model for glucocorticoid-associated osteonecrosis of the femoral head by targeted sequencing. Rheumatology (Oxford). 2022;61:846–55. https://doi.org/10.1093/rheumatology/keab394.
Liu M, et al. Sclerostin and DKK1 inhibition preserves and augments alveolar bone volume and architecture in rats with alveolar bone loss. J Dent Res. 2018;97:1031–8. https://doi.org/10.1177/0022034518766874.
Yoshimoto T, et al. Osteocytes directly regulate osteolysis via MYD88 signaling in bacterial bone infection. Nat Commun. 2022;13:6648. https://doi.org/10.1038/s41467-022-34352-z.
Leucht P, et al. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development. 2008;135:2845–54. https://doi.org/10.1242/dev.023788.
Mouraret S, et al. The potential for vertical bone regeneration via maxillary periosteal elevation. J Clin Periodontol. 2014;41:1170–7. https://doi.org/10.1111/jcpe.12310.
Li T, et al. Canonical Wnt/beta-catenin signaling has positive effects on osteogenesis, but can have negative effects on cementogenesis. J Periodontol. 2022;93:1725–37. https://doi.org/10.1002/JPER.21-0599.
Bouxsein ML, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. https://doi.org/10.1002/jbmr.141.
Yuan X, et al. Wnt-responsive stem cell fates in the oral mucosa. iScience 2019;21: 84-94. https://doi.org/10.1016/j.isci.2019.10.016.
Yuan X. et al A Wnt-Responsive PDL population effectuates extraction socket healing. J Dent Res, 2018; 22034518755719. https://doi.org/10.1177/0022034518755719.
Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397–410. https://doi.org/10.1007/BF00788278.
Dole NS, Yee CS, Schurman CA, Dallas SL, Alliston T. Assessment of osteocytes: techniques for studying morphological and molecular changes associated with perilacunar/canalicular remodeling of the bone matrix. Methods Mol Biol. 2021;2230:303–23. https://doi.org/10.1007/978-1-0716-1028-2_17.
Chan WCW, et al. Activating the unfolded protein response in osteocytes causes hyperostosis consistent with craniodiaphyseal dysplasia. Hum Mol Genet. 2017;26:4572–87. https://doi.org/10.1093/hmg/ddx339.
Acknowledgements
We thank Lauren Menke for her help in revising the manuscript and histological analysis. We thank Jonathan Joseph, Hannah Wagster, and especially McKenna Vicini for their help in histological analysis.
Author information
Authors and Affiliations
Contributions
Contributed to conception and design of the study: P.L. Cuevas, F. Aellos, I.M. Dawid, and J.A. Helms. Contributed to data acquisition, analysis, and interpretation: P.L. Cuevas, F. Aellos, I.M. Dawid, and J.A. Helms. Contributed to drafting and critical revision of the manuscript: P.L. Cuevas, F. Aellos, I.M. Dawid, and J.A. Helms. All authors gave final approval and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pedro L. Cuevas, Fabiana Aellos and Isaiah M. Dawid shared first authors.
This article is part of the Topical Collection on Osteocytes.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cuevas, P.L., Aellos, F., Dawid, I.M. et al. Wnt/β-Catenin Signaling in Craniomaxillofacial Osteocytes. Curr Osteoporos Rep 21, 228–240 (2023). https://doi.org/10.1007/s11914-023-00775-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-023-00775-w