Abstract
Purpose of Review
Osteocytes directly modify the bone surrounding the expansive lacunar-canalicular system (LCS) through both resorption and deposition. The existence of this phenomenon is now widely accepted, but is referred to as “osteocyte osteolysis,” “LCS remodeling,” and “perilacunar remodeling,” among other names. The uncertainty in naming this physiological process reflects the many persistent questions about why and how osteocytes interact with local bone matrix. The goal of this review is to examine the purpose and nature of LCS remodeling and its impacts on multiscale bone quality.
Recent Findings
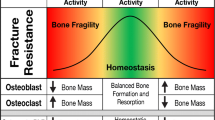
While LCS remodeling is clearly important for systemic calcium mobilization, this process may have additional potential drivers and may impact the ability of bone to resist fracture. There is abundant evidence that the osteocyte can resorb and replace bone mineral and does so outside of extreme challenges to mineral homeostasis. The impacts of the osteocyte on organic matrix are less certain, especially regarding whether osteocytes produce osteoid. Though multiple lines of evidence point towards osteocyte production of organic matrix, definitive work is needed. Recent high-resolution imaging studies demonstrate that LCS remodeling influences local material properties. The role of LCS remodeling in the maintenance and deterioration of bone matrix quality in aging and disease are active areas of research.
Summary
In this review, we highlight current progress in understanding why and how the osteocyte removes and replaces bone tissue and the consequences of these activities to bone quality. We posit that answering these questions is essential for evaluating whether, how, when, and why LCS remodeling may be manipulated for therapeutic benefit in managing bone fragility.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38.
Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2014;94(1):5–24.
Alliston T. Biological regulation of bone quality. Curr Osteoporos Rep. 2014;12(3):366–75.
• Qing H, Ardeshirpour L, Divieti Pajevic P, Dusevich V, Jähn K, Kato S, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. Journal of Bone and Mineral Research. 2012;27(5):1018–29. This seminal paper revitalized a discussion in the community regarding the existence and nature of osteocyte lacunar canalicular system remodeling and demonstrated both resorption (by increase in lacunar size) and deposition (by double fluorochrome-labeling) by osteocytes in response to lactation in mice.
Wysolmerski JJ. Osteocytes remove and replace perilacunar mineral during reproductive cycles. Bone. 2013;54(2):230–6.
• Li Y, de Bakker CMJ, Lai X, Zhao H, Parajuli A, Tseng WJ, et al. Maternal bone adaptation to mechanical loading during pregnancy, lactation, and post-weaning recovery. Bone. 2021;151. This paper demonstrates that lactation-induced LCS remodeling alters the bone response to mechanical load adaptation.
Bonewald LF, Wacker MJ. FGF23 production by osteocytes. Pediatr Nephrol. 2013;28:563–8.
Jiang YL, Wang ZX, Liu XX, Wan MD, Liu YW, Jiao B, et al. The protective effects of osteocyte-derived extracellular vesicles against Alzheimer’s disease diminished with aging. Adv Sci. 2022;2105316:1–19.
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: An endocrine cell . . . and more. Endocr Rev. 2013;34(5):658–90.
Kaya S, Basta-Pljakic J, Seref-Ferlengez Z, Majeska RJ, Cardoso L, Bromage T, et al. Lactation-induced changes in the volume of osteocyte lacunar-canalicular space alter mechanical properties in cortical bone tissue. J Bone Miner Res. 2017;32(4):688–97.
Vahidi G, Rux C, Sherk VD, Heveran CM. Lacunar-canalicular bone remodeling: impacts on bone quality and tools for assessment. Bone. 2021;143:115663.
• Rux CJ, Vahidi G, Darabi A, Cox LM, Heveran CM. Perilacunar bone tissue exhibits sub-micrometer modulus gradation which depends on the recency of osteocyte bone formation in both young adult and early-old-age female C57Bl/6 mice. Bone. 2022;157:116327. This paper showed that osteocyte lacunae are surrounded by graded bone modulus and that this gradation depends on the bone mineralization activity of individual osteocytes.
Buenzli PR, Sims NA. Quantifying the osteocyte network in the human skeleton. Bone. 2015;75:144–50.
Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009;24(4):597–605.
Kennedy OD, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone. 2014;64:132–7.
Bélanger LF. Osteocytic osteolysis. Calcif Tissue Res. 1969;4(1):1–12.
• Belanger LF, Migicovsky BB. Histochemical evidence of proteolysis in bone: the influence of parathormone. J Histochem Cytochem. 1963;11(6):734–7. This early paper established key experimental evidence of osteocytic bone resorption and, together with [Ref. 16] coined the term “osteocyte osteolysis”.
Parfitt A. The cellular basis of bone turnover and bone loss: a rebuttal of the osteocytic resorption--bone flow theory. Clin Orthop Relat Res. 1977;127:236–47.
Baylink DJ, Wergedal JE. Bone formation by osteocytes. Am J Physiol. 1971;221(3):669–78.
Mckee MD, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech. 1996;33:141–64.
Tazawa K, Hoshi K, Kawamoto S, Tanaka M, Ejiri S, Ozawa H. Osteocytic osteolysis observed in rats to which parathyroid hormone was continuously administered. J Bone Miner Metab. 2004;22(6):524–9.
Krempien B, Ritz E. Effects of parathyroid hormone on osteocytes. Ultrastructural evidence for anisotropic osteolysis and involvement of the cytoskeleton. Metab Bone Dis Relat Res. 1978;1:55–65.
Bonucci E, Gherardi G. Osteocyte ultrastructure in renal osteodystrophy. Virchows Arch A Path Anat and Histol. 1977;373:213–31.
Haller AC, Zimny ML. Effects of hibernation on interradicular alveolar bone. J Dent Res. 1977;56(12):1552–7.
Alcobendas M, Baud CA, Castanet J, Formations Squelettiques ER. Calcified tissue international structural changes of the periosteocytic area in Vipera aspis (L.) (Ophidia, Viperidae) Bone Tissue in Various Physiological Conditions. Vol. 49, Calcif Tissue Int. 1991.
Qing H, Bonewald LF. Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int J Oral Sci. 2009;1(2):59–65.
Yee CS, Schurman CA, White CR, Alliston T. Investigating Osteocytic Perilacunar/Canalicular Remodeling. Curr Osteoporos Rep. 2019;17(4):157–68.
Raisz LG. Physiology and pathophysiology of bone remodeling. Clin Chem. 1999;45(8 II):1353–8.
Hughes JM, Castellani CM, Popp KL, Guerriere KI, Matheny RW, Nindl BC, et al. The central role of osteocytes in the four adaptive pathways of bone’s mechanostat. Exerc Sport Sci Rev. 2020;48(3):140–8.
Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006:455–98.
Wysolmerski JJ. Osteocytic osteolysis: time for a second look? Bonekey Rep. 2012 Dec 5;1:229. https://doi.org/10.1038/bonekey.2012.229
• Teti A, Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone. 2009;44:11–6. This paper provides details of earlier experiments by the authors of radiolabeling collagen in egg-laying hens in a period of calcium repletion, which provides evidence of osteocyte bone matrix formation.
Gardinier JD, Al-Omaishi S, Morris MD, Kohn DH. PTH signaling mediates perilacunar remodeling during exercise. Matrix Biol. 2016;52–54:162–175.
• Jähn K, Kelkar S, Zhao H, **e Y, Tiede-Lewis LAM, Dusevich V, et al. Osteocytes acidify their microenvironment in response to pthrp in vitro and in lactating mice in vivo. J Bone Miner Res. 2017;32(8):1761–72. This paper demonstrates that osteocytes have the capability of acidifying lacunae.
Hemmatian H, Laurent MR, Bakker AD, Vanderschueren D, Klein-Nulend J, van Lenthe GH. Age-related changes in female mouse cortical bone microporosity. Bone. 2018;113(April):1–8.
Heveran CM, Rauff A, King KB, Carpenter RD, Ferguson VL. A new open-source tool for measuring 3D osteocyte lacunar geometries from confocal laser scanning microscopy reveals age-related changes to lacunar size and shape in cortical mouse bone. Bone. 2018;110:115–27.
Tiede-Lewis LM, Hulbert MA, Campos R, Dallas MR, Bonewald LF, Dallas SL. Degeneration of the osteocyte network in the C57Bl/6 mouse model of aging. Aging. 2017;9(10):2190–208.
Mazur CM, Woo JJ, Yee CS, Fields AJ, Acevedo C, Bailey KN, et al. Osteocyte dysfunction promotes osteoarthritis through MMP13-dependent suppression of subchondral bone homeostasis. Bone Res. 2019;(July):1–17.
Yajima A, Tsuchiya K, Burr DB, Minner DE, Condon KW, Miller CA, et al. Osteocytic perilacunar/canalicular turnover in hemodialysis patients with high and low serum PTH levels. Bone. 2018;113(May):68–76.
Fowler TW, Acevedo C, Mazur CM, Hall-Glenn F, Fields AJ, Bale HA, et al. Glucocorticoid suppression of osteocyte perilacunar remodeling is associated with subchondral bone degeneration in osteonecrosis. Sci Rep. 2017;7(November 2016):44618.
Britz HM, Carter Y, Jokihaara J, Leppanen O. Prolonged unloading in growing rats reduces cortical osteocyte lacunar density and volume in the distal tibia. Bone. 2012;51(5):913–9.
Blaber EA, Dvorochkin N, Lee C, Alwood JS, Yousuf R, Pianetta P, et al. Microgravity induces pelvic bone loss through osteoclastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PLoS ONE. 2013;8(4).
Lotinun S, Ishihara Y, Nagano K, Kiviranta R, Carpentier V, Neff L, et al. Cathepsin K-deficient osteocytes prevent lactation-induced bone loss and parathyroid hormone suppression. J Clin Invest. 2019;130(8):3058–71.
Emami AJ, Sebastian A, Lin YY, Yee CS, Osipov B, Loots GG, et al. Altered canalicular remodeling associated with femur fracture in mice. J Orthop Res. 2022;40(4):891–900.
Liu XS, Wang L, de Bakker CMJ, Lai X. Mechanical regulation of the maternal skeleton during reproduction and lactation. Vol. 17, Current Osteoporosis Reports. Springer; 2019. p. 375–386.
Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev. 2016;96(2):449–547.
VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, et al. Mammary-specific deletion of parathyroid hormone–related protein preserves bone mass during lactation. J Clin Investig. 2003;112(9):1429–36.
• Tang SY, Herber RP, Ho SP, Alliston T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J Bone Miner Res. 2012;27(9):1936–50. This paper demonstrates that MMP13 mediates LCS remodeling and links LCS remodeling to cortical bone matrix collagen organization and material properties.
• Dole NS, Mazur CM, Acevedo C, Ritchie RO, Mohammad KS, Alliston T, et al. Osteocyte-intrinsic TGF- b signaling regulates bone quality through perilacunar / canalicular remodeling. Cell Rep. 2017;21(9):2585–96. This paper demonstrates that impaired TGF-β signaling creates an aging-like phenotype of impaired bone fracture toughness and truncated lacunar-canalicular networks.
Kegelman CD, Coulombe JC, Jordan KM, Horan DJ, Qin L, Robling AG, et al. YAP and TAZ mediate osteocyte perilacunar/canalicular remodeling. J Bone Miner Res. 2020;35(1):196–210.
• Wang JS, Kamath T, Mazur CM, Mirzamohammadi F, Rotter D, Hojo H, et al. Control of osteocyte dendrite formation by Sp7 and its target gene osteocrin. Nat Commun. 2021;12(1). This paper demonstrates that genetic perturbation of osteocytes can lead to disrupted LCS development, but is rescuable by gene therapy, demonstrating that post-natal re-activation of LCS remodeling can restore developmental defects to the LCS. These data provide compelling evidence of a link between the developmental programs that give rise to the LCS and later LCS remodeling.
• Youlten SE, Kemp JP, Logan JG, Ghirardello EJ, Sergio CM, Dack MRG, et al. Osteocyte transcriptome map** identifies a molecular landscape controlling skeletal homeostasis and susceptibility to skeletal disease. Nat Commun. 2021;12(1). This paper demonstrates that the murine osteocyte transcriptome has signatures of both bone resorption and organic matrix formation.
Nicolella DP, Feng JQ, Moravits DE, Bonivitch AR, Wang Y, Dusecich V, et al. Effects of nanomechanical bone tissue properties on bone tissue strain: Implications for osteocyte mechanotransduction. J Musculoskelet Neuronal Interact. 2008;8(4):330–1.
Bonivtch AR, Bonewald LF, Nicolella DP. Tissue strain amplification at the osteocyte lacuna : a microstructural finite element analysis. J Biomech. 2007;40:2199–206.
Sang W, Ural A. Quantifying how altered lacunar morphology and perilacunar tissue properties influence local mechanical environment of osteocyte lacunae using finite element modeling. J Mech Behav Biomed Mater. 2022;105433.
Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. 2006;39:1735–1743.
Dole NS, Yee CS, Mazur CM, Acevedo C, Alliston T. TGFβ regulation of perilacunar/canalicular remodeling is sexually dimorphic. J Bone Miner Res. 2020;35(8):1549–61.
Schurman CA, Verbruggen SW, Alliston T. Disrupted osteocyte connectivity and pericellular fluid flow in bone with aging and defective TGF-β signaling. Proc Natl Acad Sci U S A. 2021;118(25):1–11.
Milovanovic P, Zimmermann EA, vom Scheidt A, Hoffmann B, Sarau G, Yorgan T, et al. The formation of calcified nanospherites during micropetrosis represents a unique mineralization mechanism in aged human bone. Small. 2017;13(3).
Rolvien T, Schmidt FN, Milovanovic P, Jähn K, Riedel C, Butscheidt S, et al. Early bone tissue aging in human auditory ossicles is accompanied by excessive hypermineralization, osteocyte death and micropetrosis. Sci Rep. 2018;8(1).
Milovanovic P, Busse B. Phenomenon of osteocyte lacunar mineralization: Indicator of former osteocyte death and a novel marker of impaired bone quality? Vol. 9, Endocrine Connections. BioScientifica Ltd.; 2020. p. R70–80.
Busse B, Djonic D, Milovanovic P, Hahn M, Püschel K, Ritchie RO, et al. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell. 2010;9(6):1065–75.
Ayoubi M, van Tol AF, Weinkamer R, Roschger P, Brugger PC, Berzlanovich A, et al. 3D Interrelationship between Osteocyte Network and Forming Mineral during Human Bone Remodeling. Adv Healthc Mater. 2021;10(12).
Nango N, Kubota S, Hasegawa T, Yashiro W, Momose A, Matsuo K. Osteocyte-directed bone demineralization along canaliculi. Bone. 2016;84:279–88.
• Hesse B, Varga P, Langer M, Pacureanu A, Schrof S, Männicke N, et al. Canalicular network morphology is the major determinant of the spatial distribution of mass density in human bone tissue: Evidence by means of synchrotron radiation phase-contrast nano-CT. J Bone Miner Res. 2015;30(2):346–56. This paper demonstrates bone mass gradation on the scale of hundreds of micrometers in the vicinity of lacunae and canaliculi.
Baylink DJ, Wergedal JE, Baylink J, J- D. Bone formation by osteocytes. Vol. 221, Am J Physiol. 1971.
Morrell AE, Brown GN, Robinson ST, Sattler RL, Baik AD, Zhen G, et al. Mechanically induced Ca 2 + oscillations in osteocytes release extracellular vesicles and enhance bone formation. Bone Res. 2018;(October 2017):1–11.
Hasegawa T. Ultrastructure and biological function of matrix vesicles in bone mineralization. Histochem Cell Biol. 2018;149(4):289–304.
Murshed M. Mechanism of bone mineralization. Cold Spring Harb Perspect Med. 2018:1–12.
Ruffoni D, Fratzl P, Roschger P, Klaushofer K, Weinkamer R. The bone mineralization density distribution as a fingerprint of the mineralization process. Bone. 2007;40(5):1308–19.
Donnelly E, Boskey AL, Baker SP, van der Meulen MCH. Effects of tissue age on bone tissue material composition and nanomechanical properties in the rat cortex. J Biomed Mater Res A. 2010;92(3):1048–56.
Wopenka B, Pasteris JD. A mineralogical perspective on the apatite in bone. Mater Sci Eng C. 2005;25(2):131–43.
• Kerschnitzki M, Kollmannsberger P, Burghammer M, Duda GN, Weinkamer R, Wagermaier W, et al. Architecture of the osteocyte network correlates with bone material quality. J Bone Miner Res. 2013;28(8):1837–45. This paper demonstrates that most bone mineral is located within 1 micrometer of lacunar and canalicular walls.
Zimmerman SM, Dimori M, Heard-Lipsmeyer ME, Morello R. The osteocyte transcriptome is extensively dysregulated in mouse models of osteogenesis imperfecta. JBMR Plus. 2019;3(7).
Nioi P, Taylor S, Hu R, Pacheco E, He YD, Hamadeh H, et al. Transcriptional profiling of laser capture microdissected subpopulations of the osteoblast lineage provides insight into the early response to sclerostin antibody in rats. J Bone Miner Res. 2015;30(8):1457–67.
Zambonin Zallone A, Teti A, Primavera M v, Pace G. Mature osteocytes behaviour in a repletion period: the occurrence of osteoplastic activity. Basic Appl Histochem. 1983;27(3):191–204.
Zambonin Zallone AZ, Teti A, Nico B, Primavera M. v. Osteoplastic activity of mature osteocytes evaluated by H-proline incorporation. Basic Appl Histochem. 1982;26(1):65–7.
Kamel-ElSayed SA, Tiede-Lewis LM, Lu Y, Veno PA, Dallas SL. Novel approaches for two and three dimensional multiplexed imaging of osteocytes. Bone. 2015;76:129–40.
Damrath JG, Moe SM, Wallace JM. Calcimimetics alter periosteal and perilacunar bone chronic kidney disease. J Bone Miner Res. 2022;37(7):1297–306.
Taylor EA, Donnelly E, Yao X, Johnson ML, Amugongo SK, Kimmel DB, et al. Sequential treatment of estrogen deficient, osteopenic rats with alendronate, parathyroid hormone (1–34), or raloxifene alters cortical bone mineral and matrix composition. Calcif Tissue Int. 2020;106(3):303–14.
Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2014;94:25–34.
Acknowledgements
The authors thank Ghazal Vahidi for generating confocal laser scanning microscopy images of osteocyte lacunar-canalicular networks. Funding for this work was provided through NSF CMMI2120239 (CMH); NIH RO3AG068680 (CMH); NSF CMMI1548571 (JDB), NIH R21AR071559 (JDB). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the funders.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biomechanics
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heveran, C.M., Boerckel, J.D. Osteocyte Remodeling of the Lacunar-Canalicular System: What’s in a Name?. Curr Osteoporos Rep 21, 11–20 (2023). https://doi.org/10.1007/s11914-022-00766-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-022-00766-3