Abstract
Purpose of Review
To revisit the bone tissue mechanotransduction mechanisms behind the bone tissue response to mechanical loading and, within this context, explore the possible negative influence of regular swimming practice on bone health, particularly during the growth and development period.
Recent Findings
Bone is a dynamic tissue, responsive to mechanical loading and unloading, being these adaptative responses more intense during the growth and development period. Cross-sectional studies usually report a lower bone mass in swimmers compared to athletes engaged in weigh-bearing sports. However, studies with animal models show contradictory findings about the effect of swimming on bone health, highlighting the need for longitudinal studies.
Summary
Due to its microgravity characteristics, swimming seems to impair bone mass, but mostly at the lower limbs. It is unkown if there is a causal relationship between swimming and low BMD or if other confounding factors, such as a natural selection whithin the sport, are the cause.

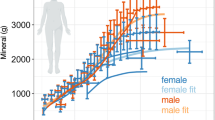
Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskelet Dis. 2016;8(6):225–35. https://doi.org/10.1177/1759720x16670154.
Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci. 2007;1116(1):281–90. https://doi.org/10.1196/annals.1402.018.
Bonewald LF. Mechanosensation and transduction in osteocytes. Bonekey Osteovision. 2006;3(10):7–15. https://doi.org/10.1138/20060233.
Qin L, Liu W, Cao H, **ao G. Molecular mechanosensors in osteocytes. Bone Res. 2020;8(8):23. https://doi.org/10.1038/s41413-020-0099-y. This review reports important evidence about the osteocytes’ role in bone mechanosensation and mechanotransduction.
Hung CT, Allen FD, Pollack SR, Brighton CT. Intracellular Ca2+ stores and extracellular Ca2+ are required in the real-time Ca2+ response of bone cells experiencing fluid flow. J Biomech. 1996;29(11):1411–7. https://doi.org/10.1016/0021-9290(96)84536-2.
Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19(4):319–38. https://doi.org/10.1615/critreveukargeneexpr.v19.i4.50.
Klein-Nulend J, van Oers RFM, Bakker AD, Bacabac RG. Nitric oxide signaling in mechanical adaptation of bone. Osteoporos Int. 2014;25(5):1427–37. https://doi.org/10.1007/s00198-013-2590-4.
Cheng B, Kato Y, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. PGE(2) is essential for gap junction-mediated intercellular communication between osteocyte-like MLO-Y4 cells in response to mechanical strain. Endocrinology. 2001;142(8):3464–73. https://doi.org/10.1210/endo.142.8.8338.
Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–15. https://doi.org/10.1016/j.bone.2007.12.224.
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24(10):1651–61. https://doi.org/10.1359/jbmr.090411.
Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali G, Fiore CE. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab. 2010;95(5):2248–53. https://doi.org/10.1210/jc.2010-0067.
Spatz JM, Fields EE, Yu EW, Divieti Pajevic P, Bouxsein ML, Sibonga JD, et al. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab. 2012;97(9):E1736–40. https://doi.org/10.1210/jc.2012-1579.
Smith SM, Heer M, Shackelford LC, Sibonga JD, Spatz J, Pietrzyk RA, Hudson EK, Zwart SR. Bone metabolism and renal stone risk during International Space Station missions. Bone. 2015;81:712–20. https://doi.org/10.1016/j.bone.2015.10.002.
Razi H, Birkhold AI, Weinkamer R, Duda GN, Willie BM, Checa S. Aging leads to a dysregulation in mechanically driven bone formation and resorption. J Bone Miner Res. 2015;30(10):1864–73. https://doi.org/10.1002/jbmr.2528.
Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26(8):1729–39. https://doi.org/10.1002/jbmr.412.
Forwood MR. Mechanical loading and the develo** skeleton. Primer Metab Bone Dis Disord Miner Metab. 2018;25:141–6. https://doi.org/10.1002/9781119266594.ch19
Faienza MF, Lassandro G, Chiarito M, Valente F, Ciaccia L, Giordano P. How physical activity across the lifespan can reduce the impact of bone ageing: a literature review. Int J Environ Res Public Health. 2020;17(6):1862. https://doi.org/10.3390/ijerph17061862. This literature review provides evidence about the importance of physical activity as a nonpharmacological therapy for prevent bone aging.
Gomez-Bruton A, Montero-Marin J, Gonzalez-Aguero A, Gomez-Cabello A, Garcia-Campayo J, Moreno LA, et al. Swimming and peak bone mineral density: a systematic review and meta-analysis. J Sports Sci. 2017;36(4):365–77. https://doi.org/10.1080/02640414.2017.1307440.
Brooke-Wavell K, Skelton DA, Barker KL, Clark EM, De Biase S, Arnold S, et al. Strong, steady and straight: UK consensus statement on physical activity and exercise for osteoporosis. Br J Sports Med. 2022;56(15):837–46. https://doi.org/10.1136/bjsports-2021-104634.
Bellver M, Del Rio L, Jovell E, Drobnic F, Trilla A. Bone mineral density and bone mineral content among female elite athletes. Bone. 2019;127:393–400. https://doi.org/10.1016/j.bone.2019.06.030. This study evidence that swimmers can display lower BMD compared with athletes of different weight-bearing sports.
Valente-Dos-Santos J, Tavares OM, Duarte JP, Sousa ESPM, Rama LM, Casanova JM, et al. Total and regional bone mineral and tissue composition in female adolescent athletes: comparison between volleyball players and swimmers. BMC Pediatr. 2018;18(1):212. https://doi.org/10.1186/s12887-018-1182-z.
Courteix D, Lespessailles E, Peres SL, Obert P, Germain P, Benhamou CL. Effect of physical training on bone mineral density in prepubertal girls: a comparative study between impact-loading and non-impact-loading sports. Osteoporos Int. 1998;8(2):152–8. https://doi.org/10.1007/bf02672512.
Creighton DL, Morgan AL, Boardley D, Brolinson PG. Weight-bearing exercise and markers of bone turnover in female athletes. J Appl Physiol. 2001;90(2):565–70. https://doi.org/10.1152/jappl.2001.90.2.565.
Fehling PC, Alekel L, Clasey J, Rector A, Stillman RJ. A comparison of bone mineral densities among female athletes in impact loading and active loading sports. Bone. 1995;17(3):205–10. https://doi.org/10.1016/8756-3282(95)00171-9.
Silva CC, Goldberg TB, Teixeira AS, Dalmas JC. The impact of different types of physical activity on total and regional bone mineral density in young brazilian athletes. J Sports Sci. 2011;29(3):227–34. https://doi.org/10.1080/02640414.2010.529456.
Gomez-Bruton A, Montero-Marin J, Gonzalez-Aguero A, Garcia-Campayo J, Moreno LA, Casajus JA, et al. The effect of swimming during childhood and adolescence on bone mineral density: a systematic review and meta-analysis. Sports Med. 2015;46:365–79. https://doi.org/10.1007/s40279-015-0427-3.
Magkos F, Yannakoulia M, Kavouras SA, Sidossis LS. The type and intensity of exercise have independent and additive effects on bone mineral density. Int J Sports Med. 2007;28(9):773–9. https://doi.org/10.1055/s-2007-964979.
Ju YI, Sone T, Ohnaru K, Tanaka K, Fukunaga M. Effect of swimming exercise on three-dimensional trabecular bone microarchitecture in ovariectomized rats. J Appl Physiol. 2015;119(9):990–7. https://doi.org/10.1152/japplphysiol.00147.2015.
Turner CH. Three rules for bone adaptation to mechanical stimuli. Bone. 1998;23(5):399–407. https://doi.org/10.1016/S8756-3282(98)00118-5.
Portier H, Benaitreau D, Pallu S. Does physical exercise always improve bone quality in rats? Life (Basel). 2020;10(10). https://doi.org/10.3390/life10100217. This review compares the effect of different exercises protocols in rats and demonstrates that swimming, the only non-weight bearing activity, is related to a higher bone negative effect.
Harding AT, Beck BR. Exercise, osteoporosis, and bone geometry. Sports (Basel). 2017;5(2):29. https://doi.org/10.3390/sports5020029.
Robling AG, Burr DB, Turner CH. Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading. J Bone Miner Res. 2000;15(8):1596–602. https://doi.org/10.1359/jbmr.2000.15.8.1596.
Gardinier JD. The diminishing returns of mechanical loading and potential mechanisms that desensitize osteocytes. Curr Osteoporos Rep. 2021;19(4):436–43. https://doi.org/10.1007/s11914-021-00693-9.
Weidauer L, Minett M, Negus C, Binkley T, Vukovich M, Wey H, Specker B. Odd-impact loading results in increased cortical area and moments of inertia in collegiate athletes. Eur J Appl Physiol. 2014;114(7):1429–38. https://doi.org/10.1007/s00421-014-2870-5.
Gómez-Bruton A, Gónzalez-Agüero A, Gómez-Cabello A, Casajús JA, Vicente-Rodríguez G. Is bone tissue really affected by swimming? A systematic review. PLoS ONE. 2013;8(8):e70119. https://doi.org/10.1371/journal.pone.0070119.
Olmedillas H, González-Agüero A, Moreno LA, Casajus JA, Vicente-Rodríguez G. Cycling and bone health: a systematic review. BMC Med. 2012;10(1):168. https://doi.org/10.1186/1741-7015-10-168.
Carbuhn AF, Fernandez TE, Bragg AF, Green JS, Crouse SF. Sport and training influence bone and body composition in women collegiate athletes. J Strength Cond Res. 2010;24(7):1710–7. https://doi.org/10.1519/JSC.0b013e3181d09eb3.
Duncan CS, Blimkie CJ, Cowell CT, Burke ST, Briody JN, Howman-Giles R. Bone mineral density in adolescent female athletes: relationship to exercise type and muscle strength. Med Sci Sports Exerc. 2002;34(2):286–94. https://doi.org/10.1097/00005768-200202000-00017.
Magkos F, Kavouras SA, Yannakoulia M, Karipidou M, Sidossi S, Sidossis LS. The bone response to non-weight-bearing exercise is sport-, site-, and sex-specific. Clin J Sport Med. 2007;17(2):123–8. https://doi.org/10.1097/JSM.0b013e318032129d.
Czeczelewski J, Dlugolecka B, Czeczelewska E, Raczynska B. Intakes of selected nutrients, bone mineralisation and density of adolescent female swimmers over a three-year period. Biol Sport. 2013;30(1):17–20. https://doi.org/10.5604/20831862.1029816.
Ferry B, Lespessailles E, Rochcongar P, Duclos M, Courteix D. Bone health during late adolescence: effects of an 8-month training program on bone geometry in female athletes. Joint Bone Spine. 2013;80(1):57–63. https://doi.org/10.1016/j.jbspin.2012.01.006.
Gomez-Bruton A, Gonzalez-Aguero A, Gomez-Cabello A, Matute-Llorente A, Casajus JA, Vicente-Rodriguez G. The effects of swimming training on bone tissue in adolescence. Scand J Med Sci Sports. 2014;25(6):589–602. https://doi.org/10.1111/sms.12378.
Kang YS, Kim SH, Kim JC. Effects of swimming exercise on high-fat diet-induced low bone mineral density and trabecular bone microstructure in rats. J Exerc Nutr Biochem. 2017;21(2):48-55. https://doi.org/10.20463/jenb.2016.0063.
Abrahin O, Rodrigues RP, Marçal AC, Alves EA, Figueiredo RC, Sousa EC. Swimming and cycling do not cause positive effects on bone mineral density: a systematic review. Rev Bras Reumatol. 2016;56(4):345–51. https://doi.org/10.1016/j.rbr.2015.09.010.
Huang T-H, Hsieh SS, Liu S-H, Chang F-L, Lin S-C, Yang R-S. Swimming training increases the post-yield energy of bone in young male rats. Calcif Tissue Int. 2010;86(2):142–53. https://doi.org/10.1007/s00223-009-9320-0.
Bourrin S, Ghaemmaghami F, Vico L, Chappard D, Gharib C, Alexandre C. Effect of a five-week swimming program on rat bone: a histomorphometric study. Calcif Tissue Int. 1992;51(2):137–42. https://doi.org/10.1007/BF00298502.
Papageorgiou M, Dolan E, Elliott-Sale KJ, Sale C. Reduced energy availability: implications for bone health in physically active populations. Eur J Nutr. 2018;57(3):847–59. https://doi.org/10.1007/s00394-017-1498-8.
Ju YI, Sone T. Effects of different types of mechanical loading on trabecular bone microarchitecture in rats. J Bone Metab. 2021;28(4):253-65. https://doi.org/10.11005/jbm.2021.28.4.253. This review updates current evidence regarding major adaptations in bone microarchitecture in small animals, supporting that different types of loading can lead to different microarchitecture adaptations.
Tan VP, Macdonald HM, Kim S, Nettlefold L, Gabel L, Ashe MC, et al. Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Miner Res. 2014;29(10):2161–81. https://doi.org/10.1002/jbmr.2254.
Nikander R, Sievänen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med Educ. 2010;8(47). https://doi.org/10.1186/1741-7015-8-47.
Hervás G, Ruiz-Litago F, Irazusta J, Irazusta A, Sanz B, Gil-Goikouria J, Fraile-Bermudez AB, Pérez-Rodrigo C, Zarrazquin I. Bone health and its relationship with impact loading and the continuity of physical activity throughout school periods. Int J Environ Res Public Health. 2019;16(16):2834. https://doi.org/10.3390/ijerph16162834. This study provides evidence about the importance of high impact physical activity to improve bone stiffness.
Fuchs RK, Bauer JJ, Snow CM. Jum** improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J Bone Miner Res. 2001;16(1):148–56. https://doi.org/10.1359/jbmr.2001.16.1.148.
Yan C, Moshage SG, Kersh ME. Play during growth: the effect of sports on bone adaptation. Curr Osteoporos Rep. 2020;18(6):684–95. https://doi.org/10.1007/s11914-020-00632-0. This study is an interesting review regarding how bone adapts to mechano-stimulation of different types of sports during growth.
Vicente-Rodríguez G. How does exercise affect bone development during growth? Sports Med. 2006;36(7):561–9. https://doi.org/10.2165/00007256-200636070-00002.
Bradbury P, Wu H, Choi JU, Rowan AE, Zhang H, Poole K, Lauko J, Chou J. Modeling the Impact of microgravity at the cellular level: implications for human disease. Front Cell Dev Biol. 2020;8:96. https://doi.org/10.3389/fcell.2020.00096. This review updates evidence related with mechanotransduction and bone physiology under microgravity environment.
Vico L, Hargens A. Skeletal changes during and after spaceflight. Nat Rev Rheumatol. 2018;14(4):229–45. https://doi.org/10.1038/nrrheum.2018.37.
Falcai MJ, Zamarioli A, Okubo R, de Paula FJ, Volpon JB. The osteogenic effects of swimming, jum**, and vibration on the protection of bone quality from disuse bone loss. Scand J Med Sci Sports. 2015;25(3):390–7. https://doi.org/10.1111/sms.12240.
Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990;5(8):843–50. https://doi.org/10.1002/jbmr.5650050807.
Beller G, Belavý DL, Sun L, Armbrecht G, Alexandre C, Felsenberg D. WISE-2005: bed-rest induced changes in bone mineral density in women during 60 days simulated microgravity. Bone. 2011;49(4):858–66. https://doi.org/10.1016/j.bone.2011.06.021.
Armbrecht G, Belavý DL, Backström M, Beller G, Alexandre C, Rizzoli R, Felsenberg D. Trabecular and cortical bone density and architecture in women after 60 days of bed rest using high-resolution pQCT: WISE 2005. J Bone Miner Res. 2011;26(10):2399–410. https://doi.org/10.1002/jbmr.482.
Sibonga JD, Evans HJ, Sung HG, Spector ER, Lang TF, Oganov VS, Bakulin AV, Shackelford LC, LeBlanc AD. Recovery of spaceflight-induced bone loss: bone mineral density after long-duration missions as fitted with an exponential function. Bone. 2007;41(6):973–8. https://doi.org/10.1016/j.bone.2007.08.022.
Orwoll ES, Adler RA, Amin S, Binkley N, Lewiecki EM, Petak SM, Shapses SA, Sinaki M, Watts NB, Sibonga JD. Skeletal health in long-duration astronauts: nature, assessment, and management recommendations from the NASA Bone Summit. J Bone Miner Res. 2013;28(6):1243–55. https://doi.org/10.1002/jbmr.1948.
LeBlanc A, Schneider V, Shackelford L, West S, Oganov V, Bakulin A, et al. Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Nueronal Interact. 2000;1(2):157–60.
Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz-Snyder L, Zwart SR. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: evidence from biochemistry and densitometry. J Bone Miner Res. 2012;27(9):1896–906. https://doi.org/10.1002/jbmr.1647.
Sibonga J, Matsumoto T, Jones J, Shapiro J, Lang T, Shackelford L, Smith SM, Young M, Keyak J, Kohri K, Ohshima H, Spector E, LeBlanc A. Resistive exercise in astronauts on prolonged spaceflights provides partial protection against spaceflight-induced bone loss. Bone. 2019;128:112037. https://doi.org/10.1016/j.bone.2019.07.013.
Midura RJ, Dillman CJ, Grabiner MD. Low amplitude, high frequency strains imposed by electrically stimulated skeletal muscle retards the development of osteopenia in the tibiae of hindlimb suspended rats. Med Eng Phys. 2005;27(4):285–93. https://doi.org/10.1016/j.medengphy.2004.12.014.
Vickerton P, Jarvis JC, Gallagher JA, Akhtar R, Sutherland H, Jeffery N. Morphological and histological adaptation of muscle and bone to loading induced by repetitive activation of muscle. Proc R Soc B. 2014;281(1788):20140786. https://doi.org/10.1098/rspb.2014.0786.
Robling AG. Is bone's response to mechanical signals dominated by muscle forces? Med Sci Sports Exerc. 2009;41(11):2044–9. https://doi.org/10.1249/MSS.0b013e3181a8c702.
LeBlanc AD, Schneider VS, Evans HJ, Pientok C, Rowe R, Spector E. Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol. 1992;73(5):2172–8. https://doi.org/10.1152/jappl.1992.73.5.2172.
Gordon CM, Zemel BS, Wren TA, Leonard MB, Bachrach LK, Rauch F, Gilsanz V, Rosen CJ, Winer KK. The determinants of peak bone mass. J Pediatr. 2016;180:261–9. https://doi.org/10.1016/j.jpeds.2016.09.056.
Ramos E, Frontera WR, Llopart A, Feliciano D. Muscle strength and hormonal levels in adolescents: gender related differences. Int J Sports Med. 1998;19(08):526–31.
Zhu X, Zheng H. Factors influencing peak bone mass gain. Front Med. 2021;15(1):53–69. https://doi.org/10.1007/s11684-020-0748-y. This review updates available information about the peak bone mass and its determinants, such as the importance of the physical activity practice during the bone growth and development period.
Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O’Karma M, Wallace TC, Zemel BS. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281–386. https://doi.org/10.1007/s00198-015-3440-3.
Rizzoli R, Bianchi ML, Garabédian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46(2):294–305. https://doi.org/10.1016/j.bone.2009.10.005.
Faulkner RA, Bailey DA. Osteoporosis: a pediatric concern? J Sports Sci Med. 2007;51:1–12. https://doi.org/10.1159/000102993.
Xue S, Kemal O, Lu M, Lix LM, Leslie WD, Yang S. Age at attainment of peak bone mineral density and its associated factors: The National Health and Nutrition Examination Survey 2005-2014. Bone. 2020;131:115163. https://doi.org/10.1016/j.bone.2019.115163.
Boot AM, de Ridder MA, van der Sluis IM, van Slobbe I, Krenning EP, Keizer-Schrama SM. Peak bone mineral density, lean body mass and fractures. Bone. 2010;46(2):336–41. https://doi.org/10.1016/j.bone.2009.10.003.
Bonjour JP, Chevalley T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr Rev. 2014;35(5):820–47. https://doi.org/10.1210/er.2014-1007.
Karlsson MK, Rosengren BE. Exercise and peak bone mass. Curr Osteoporos Rep. 2020;18(3):285–90. https://doi.org/10.1007/s11914-020-00588-1.
McCormack SE, Cousminer DL, Chesi A, Mitchell JA, Roy SM, Kalkwarf HJ, Lappe JM, Gilsanz V, Oberfield SE, Shepherd JA, Winer KK, Kelly A, Grant SFA, Zemel BS. Association between linear growth and bone accrual in a diverse cohort of children and adolescents. JAMA Pediatr Sci Child Adolesc Health. 2017;171(9):e171769. https://doi.org/10.1001/jamapediatrics.2017.1769.
Callewaert F, Venken K, Kopchick JJ, Torcasio A, van Lenthe GH, Boonen S, Vanderschueren D. Sexual dimorphism in cortical bone size and strength but not density is determined by independent and time-specific actions of sex steroids and IGF-1: evidence from pubertal mouse models. J Bone Miner Res. 2010;25(3):617–26. https://doi.org/10.1359/jbmr.090828.
Turner CH, Robling AG. Designing exercise regimens to increase bone strength. Exerc Sport Sci Rev. 2003;31(1):45–50.
Maggiano IS, Maggiano CM, Tiesler VG, Chi-Keb JR, Stout SD. Drifting diaphyses: asymmetry in diametric growth and adaptation along the humeral and femoral length. Anat Rec (Hoboken). 2015;298(10):1689–99. https://doi.org/10.1002/ar.23201.
Isojima T, Sims NA. Cortical bone development, maintenance and porosity: genetic alterations in humans and mice influencing chondrocytes, osteoclasts, osteoblasts and osteocytes. Cell Mol Life Sci. 2021;78(15):5755–73. https://doi.org/10.1007/s00018-021-03884-w.
Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton LJ 3rd. Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res. 2006;21(1):124–31. https://doi.org/10.1359/jbmr.050916.
Riggs BL, Melton Iii LJ 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 2004;19(12):1945-1954. https://doi.org/10.1359/jbmr.040916.
McVeigh JA, Howie EK, Zhu K, Walsh JP, Straker L. Organized sport participation from childhood to adolescence is associated with bone mass in young adults from the Raine Study. J Bone Miner Res. 2019;34(1):67–74. https://doi.org/10.1002/jbmr.3583.
Gabel L, Macdonald HM, Nettlefold L, McKay HA. Physical activity, sedentary time, and bone strength from childhood to early adulthood: a mixed longitudinal HR-pQCT study. J Bone Miner Res. 2017;32(7):1525–36. https://doi.org/10.1002/jbmr.3115.
Palaiothodorou D, Antoniou T, Vagenas G. Bone asymmetries in the limbs of children tennis players: testing the combined effects of age, sex, training time, and maturity status. J Sports Sci. 2020;38(20):2298–306. https://doi.org/10.1080/02640414.2020.1779490.
Warden SJ, Mantila Roosa SM, Kersh ME, Hurd AL, Fleisig GS, Pandy MG, Fuchs RK. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci. 2014;111(14):5337–42. https://doi.org/10.1073/pnas.1321605111.
Nilsson M, Ohlsson C, Mellström D, Lorentzon M. Previous sport activity during childhood and adolescence is associated with increased cortical bone size in young adult men. J Bone Miner Res. 2009;24(1):125–33. https://doi.org/10.1359/jbmr.080909.
Warden SJ, Fuchs RK, Castillo AB, Nelson IR, Turner CH. Exercise when young provides lifelong benefits to bone structure and strength. J Bone Miner Res. 2007;22(2):251–9. https://doi.org/10.1359/jbmr.061107.
Emslander HC, Sinaki M, Muhs JM, Chao EY, Wahner HW, Bryant SC, et al. Bone mass and muscle strength in female college athletes (runners and swimmers). Mayo Clin Proc. 1998;73(12):1151–60. https://doi.org/10.4065/73.12.1151.
Min SK, Oh T, Kim SH, Cho J, Chung HY, Park DH, et al. Position statement: exercise guidelines to increase peak bone mass in adolescents. J Bone Metab. 2019;26(4):225-39. https://doi.org/10.11005/jbm.2019.26.4.225. This position statement updates the guidelines to increase the peak bone mass in adolescents and includes osteogenic exercises performance in their daily physical activities’ recommendations.
Gomez-Bruton A, Gonzalez-Aguero A, Matute-Llorente A, Lozano-Berges G, Gomez-Cabello A, Moreno LA, Casajus JA, Vicente-Rodríguez G. The muscle-bone unit in adolescent swimmers. Osteoporos Int. 2019;30(5):1079–88. https://doi.org/10.1007/s00198-019-04857-3.
Gómez-Bruton A, González-Agüero A, Gómez-Cabello A, Matute-Llorente A, Casajús JA, Vicente-Rodríguez G. Swimming and bone: is low bone mass due to hypogravity alone or does other physical activity influence it? Osteoporos Int. 2016;27(5):1785–93. https://doi.org/10.1007/s00198-015-3448-8.
Lee EJ, Long KA, Risser WL, Poindexter HB, Gibbons WE, Goldzieher J. Variations in bone status of contralateral and regional sites in young athletic women. Med Sci Sports Exerc. 1995;27(10):1354–61.
Derman O, Cinemre A, Kanbur N, Dogan M, Kilic M, Karaduman E. Effect of swimming on bone metabolism in adolescents. Turk J Pediatr. 2008;50(2):149–54.
Taaffe DR, Snow-Harter C, Connolly DA, Robinson TL, Brown MD, Marcus R. Differential effects of swimming versus weight-bearing activity on bone mineral status of eumenorrheic athletes. J Bone Miner Res. 1995;10(4):586–93. https://doi.org/10.1002/jbmr.5650100411.
Agostinete RR, Duarte JP, Valente-Dos-Santos J, Coelho ESMJ, Tavares OM, Conde JM, et al. Bone tissue, blood lipids and inflammatory profiles in adolescent male athletes from sports contrasting in mechanical load. PLoS ONE. 2017;12(6):1–18. https://doi.org/10.1371/journal.pone.0180357.
Gruodyte R, Jurimae J, Saar M, Jurimae T. The relationships among bone health, insulin-like growth factor-1 and sex hormones in adolescent female athletes. J Bone Miner Metab. 2010;28(3):306–13. https://doi.org/10.1007/s00774-009-0130-2.
Agostinete RR, Maillane-Vanegas S, Lynch KR, Turi-Lynch B, Coelho ESMJ, Campos EZ, et al. The impact of training load on bone mineral density of adolescent swimmers: a structural equation modeling approach. Pediatr Exerc Sci. 2017;29(4):520–8. https://doi.org/10.1123/pes.2017-0008.
Vantorre J, Seifert L, Fernandes RJ, Vilas-Boas JP, Chollet D. Kinematical profiling of the front crawl start. Int J Sports Med. 2010;31(1):16–21. https://doi.org/10.1055/s-0029-1241208.
Chainok P, Machado L, de Jesus K, Abraldes JA, Borgonovo-Santos M, Fernandes RJ, Vilas-Boas JP. Backstroke to breaststroke turning performance in age-group swimmers: hydrodynamic characteristics and pull-out strategy. Int J Environ Res Public Health. 2021;18(1858):1–10. https://doi.org/10.3390/ijerph18041858.
Figueiredo P, Rouard A, Vilas-Boas JP, Fernandes RJ. Upper- and lower-limb muscular fatigue during the 200-m front crawl. Appl Physiol Nutr Metab. 2013;38(7):716–24. https://doi.org/10.1139/apnm-2012-0263.
Hart NH, Nimphius S, Rantalainen T, Ireland A, Siafarikas A, Newton RU. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J Musculoskelet Neuronal Interact. 2017;17(3):114–39.
Maillane-Vanegas S, Agostinete RR, Lynch KR, Ito IH, Luiz-de-Marco R, Rodrigues-Junior MA, Turi-Lynch BC, Fernandes RA. Bone mineral density and sports participation. J Clin Densitom. 2020;23(2):294–302. https://doi.org/10.1016/j.jocd.2018.05.041.
Vlachopoulos D, Barker AR, Ubago-Guisado E, Ortega FB, Krustrup P, Metcalf B, Castro Pinero J, Ruiz JR, Knapp KM, Williams CA, Moreno LA, Gracia-Marco L. The effect of 12-month participation in osteogenic and non-osteogenic sports on bone development in adolescent male athletes. The PRO-BONE study. J Sci Med Sport. 2018;21(4):404–9. https://doi.org/10.1016/j.jsams.2017.08.018.
Maimoun L, Coste O, Philibert P, Briot K, Mura T, Galtier F, et al. Peripubertal female athletes in high-impact sports show improved bone mass acquisition and bone geometry. Metabolism. 2013;62(8):1088–98. https://doi.org/10.1016/j.metabol.2012.11.010.
Dias Quiterio AL, Carnero EA, Baptista FM, Sardinha LB. Skeletal mass in adolescent male athletes and nonathletes: relationships with high-impact sports. J Strength Cond Res. 2011;25(12):3439–47.
Vlachopoulos D, Barker AR, Williams CA, SA AR, Knapp KM, Metcalf BS, et al. The impact of sport participation on bone mass and geometry in male adolescents. Med Sci Sports Exerc. 2017;49(2):317–26. https://doi.org/10.1249/mss.0000000000001091.
Agostinete RR, Lynch KR, Gobbo LA, Lima MC, Ito IH, Luiz-de-Marco R, et al. Basketball affects bone mineral density accrual in boys more than swimming and other impact sports: 9-mo follow-up. J Clin Densitom. 2016;19(3):375–81. https://doi.org/10.1016/j.jocd.2016.04.006.
Ribeiro-Dos-Santos MR, Lynch KR, Agostinete RR, Maillane-Vanegas S, Turi-Lynch B, Ito IH, et al. Prolonged practice of swimming is negatively related to bone mineral density gains in adolescents. J Bone Metab. 2016;23(3):149-55. https://doi.org/10.11005/jbm.2016.23.3.149.
Maimoun L, Coste O, Philibert P, Briot K, Mura T, Galtier F, et al. Testosterone secretion in elite adolescent swimmers does not modify bone mass acquisition: a 1-year follow-up study. Fertil Steril. 2013;99(1):270–8. https://doi.org/10.1016/j.fertnstert.2012.08.020.
Pezhman L, Sheikhzadeh Hesari F, Ghiasi R, Alipour MR. The impact of forced swimming on expression of RANKL and OPG in a type 2 diabetes mellitus rat model. Arch Physiol Biochem. 2019;125(3):195–200. https://doi.org/10.1080/13813455.2018.1446178.
Snyder A, Zierath JR, Hawley JA, Sleeper MD, Craig BW. The effects of exercise mode, swimming vs. running, upon bone growth in the rapidly growing female rat. Mech Ageing Dev. 1992;66(1):59–69. https://doi.org/10.1016/0047-6374(92)90073-M.
Gomes GJ, Carlo RJD, Silva MFD, Cunha D, Silva ED, Silva KAD, et al. Swimming training potentiates the recovery of femoral neck strength in young diabetic rats under insulin therapy. Clinics. 2019;74:e829. https://doi.org/10.6061/clinics/2019/e829.
Swissa-Sivan A, Azoury R, Statter M, Leichter I, Nyska A, Nyska M, Menczel J, Samueloff S. The effect of swimming on bone modeling and composition in young adult rats. Calcif Tissue Int. 1990;47(3):173–7. https://doi.org/10.1007/BF02555984.
Ju Y-I, Sone T, Ohnaru K, Tanaka K, Yamaguchi H, Fukunaga M. Effects of different types of jump impact on trabecular bone mass and microarchitecture in growing rats. PLoS ONE. 2014;9(9):e107953. https://doi.org/10.1371/journal.pone.0107953.
Ju YI, Sone T, Ohnaru K, Choi HJ, Choi KA, Fukunaga M. Jump exercise during hindlimb unloading protect against the deterioration of trabecular bone microarchitecture in growing young rats. Springerplus. 2013;2(1):35. https://doi.org/10.1186/2193-1801-2-35.
Hemmatian H, Bakker AD, Klein-Nulend J, van Lenthe GH. Alterations in osteocyte lacunar morphology affect local bone tissue strains. J Mech Behav Biomed Mater. 2021;123:104730. https://doi.org/10.1016/j.jmbbm.2021.104730.
Funding
The authors perform their research activity at the Research Center in Physical Activity, Health and Leisure (CIAFEL), Faculty of Sport, University of Porto (FADEUP) which is funded by Fundação Para a Ciência e Tecnologia (FCT) grant UIDB/00617/2020 and at the Laboratory for Integrative and Translational Research in Population Health (ITR) which is funded by FCT grant LA/P/0064/2020. The author’s work is currently supported by FCT grant PTDC/SAU-DES/4113/2020.
Author information
Authors and Affiliations
Contributions
Conceptualization, H.F. and A.B.; investigation, A.B., L.F. and L.M.; resources, H.F.; writing—original draft preparation, A.B., L.F. and L.M.; writing—review and editing, A.B. and H.F.; supervision, H.F.; funding acquisition, H.F. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Humans and Animal Rights
All reported studies/experiments with human or animal subjects performed by the author have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nutrition, Exercise and Lifestyle
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bezerra, A., Freitas, L., Maciel, L. et al. Bone Tissue Responsiveness To Mechanical Loading—Possible Long-Term Implications of Swimming on Bone Health and Bone Development. Curr Osteoporos Rep 20, 453–468 (2022). https://doi.org/10.1007/s11914-022-00758-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-022-00758-3