Abstract
Purpose of Review
To summarize the key factors contributing to the onset and progress of nonalcoholic fatty liver disease (NAFLD) and put them in a system genetics context. We particularly focus on how genetic regulation of hepatic lipids contributes to NAFLD.
Recent Findings
NAFLD is characterized by excessive accumulation of fat in the liver. This can progress to steatohepatitis (inflammation and hepatocyte injury) and eventually, cirrhosis. The severity of NAFLD is determined by a combination of factors including obesity, insulin resistance, and lipotoxic lipids, along with genetic susceptibility. Numerous studies have been conducted on large human cohorts and mouse panels, to identify key determinants in the genome, transcriptome, proteome, lipidome, microbiome and different environmental conditions contributing to NAFLD.
Summary
We review common factors contributing to NAFLD and put them in a systems genetics context. In particular, we describe how genetic regulation of liver lipids contributes to NAFLD. The combination of an unhealthy lifestyle and genetic predisposition increases the likelihood of accumulating lipotoxic specie lipids that may be one of the driving forces behind develo** severe forms of NAFLD.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Roeb E, Steffen HM, Bantel H, Baumann U, Canbay A, Demir M, et al. S2k Guideline non-alcoholic fatty liver disease. Z Gastroenterol. 2015;53(7):668–723. https://doi.org/10.1055/s-0035-1553193.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. https://doi.org/10.1002/hep.29367.
Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53(2):372–84. https://doi.org/10.1016/j.jhep.2010.04.008.
Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–33. https://doi.org/10.1002/hep.29466.
Ahmed EA, El-Derany MO, Anwar AM, Saied EM, Magdeldin S. Metabolomics and lipidomics screening reveal reprogrammed signaling pathways toward cancer development in non-alcoholic steatohepatitis. Int J Mol Sci. 2022;24(1). https://doi.org/10.3390/ijms24010210.
Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–705. https://doi.org/10.1136/gutjnl-2020-320622.
Przybyszewski EM, Targher G, Roden M, Corey KE. Nonalcoholic fatty liver disease and cardiovascular disease. Clin Liver Dis (Hoboken). 2021;17(1):19–22. https://doi.org/10.1002/cld.1017.
Kasper P, Martin A, Lang S, Kutting F, Goeser T, Demir M, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110(7):921–37. https://doi.org/10.1007/s00392-020-01709-7.
Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10(6):646–50. https://doi.org/10.1016/j.cgh.2011.12.039.
Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. https://doi.org/10.1136/bmj.d6891.
Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41(2):372–9. https://doi.org/10.1002/hep.20554.
Anstee QM, Daly AK, Day CP. Genetic modifiers of non-alcoholic fatty liver disease progression. Biochim Biophys Acta. 2011;1812(11):1557–66. https://doi.org/10.1016/j.bbadis.2011.07.017.
Cai B, Dongiovanni P, Corey KE, Wang X, Shmarakov IO, Zheng Z, et al. Macrophage MerTK promotes liver fibrosis in nonalcoholic steatohepatitis. Cell Metab. 2020;31(2):406-21 e7. https://doi.org/10.1016/j.cmet.2019.11.013.
Dongiovanni P, Stender S, Pietrelli A, Mancina RM, Cespiati A, Petta S, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283(4):356–70. https://doi.org/10.1111/joim.12719.
Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65(8):1049–61. https://doi.org/10.1016/j.metabol.2016.02.014.
Lonardo A, Nascimbeni F, Maurantonio M, Marrazzo A, Rinaldi L, Adinolfi LE. Nonalcoholic fatty liver disease: evolving paradigms. World J Gastroenterol. 2017;23(36):6571–92. https://doi.org/10.3748/wjg.v23.i36.6571.
Musso G, Cassader M, Paschetta E, Gambino R. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology. 2018;155(2):282-302 e8. https://doi.org/10.1053/j.gastro.2018.06.031.
Seldin M, Yang X, Lusis AJ. Systems genetics applications in metabolism research. Nat Metab. 2019;1(11):1038–50. https://doi.org/10.1038/s42255-019-0132-x.
Jha P, McDevitt MT, Gupta R, Quiros PM, Williams EG, Gariani K, et al. Systems analyses reveal physiological roles and genetic regulators of liver lipid species. Cell Syst. 2018;6(6):722-33 e6. https://doi.org/10.1016/j.cels.2018.05.016.
Parker BL, Calkin AC, Seldin MM, Keating MF, Tarling EJ, Yang P, et al. An integrative systems genetic analysis of mammalian lipid metabolism. Nature. 2019;567(7747):187–93. https://doi.org/10.1038/s41586-019-0984-y.
Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46(4):352–6. https://doi.org/10.1038/ng.2901.
Taliento AE, Dallio M, Federico A, Prati D, Valenti L. Novel insights into the genetic landscape of nonalcoholic fatty liver disease. Int J Environ Res Public Health. 2019;16(15). https://doi.org/10.3390/ijerph16152755.
Sevastianova K, Kotronen A, Gastaldelli A, Perttila J, Hakkarainen A, Lundbom J, et al. Genetic variation in PNPLA3 (adiponutrin) confers sensitivity to weight loss-induced decrease in liver fat in humans. Am J Clin Nutr. 2011;94(1):104–11. https://doi.org/10.3945/ajcn.111.012369.
Norum KR, Berg T, Helgerud P, Drevon CA. Transport of cholesterol. Physiol Rev. 1983;63(4):1343–419. https://doi.org/10.1152/physrev.1983.63.4.1343.
Saponaro C, Gaggini M, Carli F, Gastaldelli A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients. 2015;7(11):9453–74. https://doi.org/10.3390/nu7115475.
Liu Q, Bengmark S, Qu S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2010;9:42. https://doi.org/10.1186/1476-511X-9-42.
Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010;2010:513948. https://doi.org/10.1155/2010/513948.
Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–89. https://doi.org/10.1002/hep.23280.
Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, et al. NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35(2):373–9. https://doi.org/10.1053/jhep.2002.30692.
Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91(12):4753–61. https://doi.org/10.1210/jc.2006-0587.
Parks BW, Nam E, Org E, Kostem E, Norheim F, Hui ST, et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013;17(1):141–52. https://doi.org/10.1016/j.cmet.2012.12.007.
Brahe LK, Astrup A, Larsen LH. Can we prevent obesity-related metabolic diseases by dietary modulation of the gut microbiota? Adv Nutr. 2016;7(1):90–101. https://doi.org/10.3945/an.115.010587.
He M, Shi B. Gut microbiota as a potential target of metabolic syndrome: the role of probiotics and prebiotics. Cell Biosci. 2017;7:54. https://doi.org/10.1186/s13578-017-0183-1.
Wortelboer K, Nieuwdorp M, Herrema H. Fecal microbiota transplantation beyond Clostridioides difficile infections. EBioMedicine. 2019;44:716–29. https://doi.org/10.1016/j.ebiom.2019.05.066.
Hui ST, Kurt Z, Tuominen I, Norheim F, Davis RC, Pan C, et al. The genetic architecture of diet-induced hepatic fibrosis in mice. Hepatology. 2018;68(6):2182–96. https://doi.org/10.1002/hep.30113.
Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13(7):412–25. https://doi.org/10.1038/nrgastro.2016.85.
Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48(2):206–11. https://doi.org/10.1136/gut.48.2.206.
Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–6. https://doi.org/10.1038/nature11400.
Mazagova M, Wang L, Anfora AT, Wissmueller M, Lesley SA, Miyamoto Y, et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. 2015;29(3):1043–55. https://doi.org/10.1096/fj.14-259515.
Fang J, Yu CH, Li XJ, Yao JM, Fang ZY, Yoon SH, et al. Gut dysbiosis in nonalcoholic fatty liver disease: pathogenesis, diagnosis, and therapeutic implications. Front Cell Infect Microbiol. 2022;12:997018. https://doi.org/10.3389/fcimb.2022.997018.
Carr RM, Reid AE. FXR agonists as therapeutic agents for non-alcoholic fatty liver disease. Curr Atheroscler Rep. 2015;17(4):500. https://doi.org/10.1007/s11883-015-0500-2.
Khalid Q, Bailey I, Patel VB. Non-alcoholic fatty liver disease: the effect of bile acids and farnesoid X receptor agonists on pathophysiology and treatment. Liver Res - Open J. 2015;1(2):32–40. https://doi.org/10.17140/lroj-1-106.
Hikida RS, Staron RS, Hagerman FC, Sherman WM, Costill DL. Muscle fiber necrosis associated with human marathon runners. J Neurol Sci. 1983;59(2):185–203.
Nagashimada M, Ota T. Role of vitamin E in nonalcoholic fatty liver disease. IUBMB Life. 2019;71(4):516–22. https://doi.org/10.1002/iub.1991.
Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018;2018:9547613. https://doi.org/10.1155/2018/9547613.
DelliBovi AP, Marciano F, Mandato C, Siano MA, Savoia M, Vajro P. Oxidative stress in non-alcoholic fatty liver disease. An updated mini review. Front Med (Lausanne). 2021;8:595371. https://doi.org/10.3389/fmed.2021.595371.
Kawahara H, Fukura M, Tsuchishima M, Takase S. Mutation of mitochondrial DNA in livers from patients with alcoholic hepatitis and nonalcoholic steatohepatitis. Alcohol Clin Exp Res. 2007;31(1 Suppl):S54-60. https://doi.org/10.1111/j.1530-0277.2006.00287.x.
Persico M, Masarone M, Damato A, Ambrosio M, Federico A, Rosato V, et al. Non alcoholic fatty liver disease and eNOS dysfunction in humans. BMC Gastroenterol. 2017;17(1):35. https://doi.org/10.1186/s12876-017-0592-y.
Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37(3):544–50. https://doi.org/10.1053/jhep.2003.50095.
El-Koofy NM, El-Karaksy HM, Mandour IM, Anwar GM, El-Raziky MS, El-Hennawy AM. Genetic polymorphisms in non-alcoholic fatty liver disease in obese Egyptian children. Saudi J Gastroenterol. 2011;17(4):265–70. https://doi.org/10.4103/1319-3767.82582.
Namikawa C, Shu-** Z, Vyselaar JR, Nozaki Y, Nemoto Y, Ono M, et al. Polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in non-alcoholic steatohepatitis. J Hepatol. 2004;40(5):781–6. https://doi.org/10.1016/j.jhep.2004.01.028.
Nobili V, Donati B, Panera N, Vongsakulyanon A, Alisi A, Dallapiccola B, et al. A 4-polymorphism risk score predicts steatohepatitis in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2014;58(5):632–6. https://doi.org/10.1097/MPG.0000000000000279.
Varela NM, Quinones LA, Orellana M, Poniachik J, Csendes A, Smok G, et al. Study of cytochrome P450 2E1 and its allele variants in liver injury of nondiabetic, nonalcoholic steatohepatitis obese women. Biol Res. 2008;41(1):81–92.
Krawczyk M, Liebe R, Lammert F. Toward genetic prediction of nonalcoholic fatty liver disease trajectories: PNPLA3 and beyond. Gastroenterology. 2020;158(7):1865-80 e1. https://doi.org/10.1053/j.gastro.2020.01.053.
Eslam M, Sanyal AJ, George J, International Consensus P. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999-2014 e1. https://doi.org/10.1053/j.gastro.2019.11.312.
Al-Serri A, Anstee QM, Valenti L, Nobili V, Leathart JB, Dongiovanni P, et al. The SOD2 C47T polymorphism influences NAFLD fibrosis severity: evidence from case-control and intra-familial allele association studies. J Hepatol. 2012;56(2):448–54. https://doi.org/10.1016/j.jhep.2011.05.029.
Smagris E, BasuRay S, Li J, Huang Y, Lai KM, Gromada J, et al. Pnpla3I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology. 2015;61(1):108–18. https://doi.org/10.1002/hep.27242.
BasuRay S, Wang Y, Smagris E, Cohen JC, Hobbs HH. Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc Natl Acad Sci U S A. 2019;116(19):9521–6. https://doi.org/10.1073/pnas.1901974116.
**itore P, Pirazzi C, Mancina RM, Motta BM, Indiveri C, Pujia A, et al. Recombinant PNPLA3 protein shows triglyceride hydrolase activity and its I148M mutation results in loss of function. Biochim Biophys Acta. 2014;1841(4):574–80. https://doi.org/10.1016/j.bbalip.2013.12.006.
Kumari M, Schoiswohl G, Chitraju C, Paar M, Cornaciu I, Rangrez AY, et al. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 2012;15(5):691–702. https://doi.org/10.1016/j.cmet.2012.04.008.
He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285(9):6706–15. https://doi.org/10.1074/jbc.M109.064501.
Romeo S, Kozlitina J, **ng C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–5. https://doi.org/10.1038/ng.257.
Basantani MK, Sitnick MT, Cai L, Brenner DS, Gardner NP, Li JZ, et al. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J Lipid Res. 2011;52(2):318–29. https://doi.org/10.1194/jlr.M011205.
Luukkonen PK, Nick A, Holtta-Vuori M, Thiele C, Isokuortti E, Lallukka-Bruck S, et al. Human PNPLA3-I148M variant increases hepatic retention of polyunsaturated fatty acids. JCI Insight. 2019;4(16). https://doi.org/10.1172/jci.insight.127902.
Lu Y, Feng T, Zhao J, Jiang P, Xu D, Zhou M, et al. Polyene phosphatidylcholine ameliorates high fat diet-induced non-alcoholic fatty liver disease via remodeling metabolism and inflammation. Front Physiol. 2022;13:810143. https://doi.org/10.3389/fphys.2022.810143.
Maev IV, Samsonov AA, Palgova LK, Pavlov CS, Shirokova EN, Vovk EI, et al. Effectiveness of phosphatidylcholine as adjunctive therapy in improving liver function tests in patients with non-alcoholic fatty liver disease and metabolic comorbidities: real-life observational study from Russia. BMJ Open Gastroenterol. 2020;7(1):e000368. https://doi.org/10.1136/bmjgast-2019-000368.
Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61(1):75–81. https://doi.org/10.1016/j.jhep.2014.02.030.
Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53(6):1883–94. https://doi.org/10.1002/hep.24283.
Smagris E, Gilyard S, BasuRay S, Cohen JC, Hobbs HH. Inactivation of Tm6sf2, a gene defective in fatty liver disease, impairs lipidation but not secretion of very low density lipoproteins. J Biol Chem. 2016;291(20):10659–76. https://doi.org/10.1074/jbc.M116.719955.
Li XY, Liu Z, Li L, Wang HJ, Wang H. TM6SF2 rs58542926 is related to hepatic steatosis, fibrosis and serum lipids both in adults and children: a meta-analysis. Front Endocrinol (Lausanne). 2022;13:1026901. https://doi.org/10.3389/fendo.2022.1026901.
Fan Y, Lu H, Guo Y, Zhu T, Garcia-Barrio MT, Jiang Z, et al. Hepatic transmembrane 6 superfamily member 2 regulates cholesterol metabolism in mice. Gastroenterology. 2016;150(5):1208–18. https://doi.org/10.1053/j.gastro.2016.01.005.
Mahdessian H, Taxiarchis A, Popov S, Silveira A, Franco-Cereceda A, Hamsten A, et al. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proc Natl Acad Sci U S A. 2014;111(24):8913–8. https://doi.org/10.1073/pnas.1323785111.
Ehrhardt N, Doche ME, Chen S, Mao HZ, Walsh MT, Bedoya C, et al. Hepatic Tm6sf2 overexpression affects cellular ApoB-trafficking, plasma lipid levels, hepatic steatosis and atherosclerosis. Hum Mol Genet. 2017;26(14):2719–31. https://doi.org/10.1093/hmg/ddx159.
Gronert K, Kantarci A, Levy BD, Clish CB, Odparlik S, Hasturk H, et al. A molecular defect in intracellular lipid signaling in human neutrophils in localized aggressive periodontal tissue damage. J Immunol. 2004;172(3):1856–61. https://doi.org/10.4049/jimmunol.172.3.1856.
Koybasi S, Senkal CE, Sundararaj K, Spassieva S, Bielawski J, Osta W, et al. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem. 2004;279(43):44311–9. https://doi.org/10.1074/jbc.M406920200.
Kroesen BJ, Pettus B, Luberto C, Busman M, Sietsma H, de Leij L, et al. Induction of apoptosis through B-cell receptor cross-linking occurs via de novo generated C16-ceramide and involves mitochondria. J Biol Chem. 2001;276(17):13606–14. https://doi.org/10.1074/jbc.M009517200.
Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology (Baltimore, MD). 2007;45(6):1366–74. https://doi.org/10.1002/hep.21655.
Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68(2):280–95. https://doi.org/10.1016/j.jhep.2017.11.014.
Brankovic M, Jovanovic I, Dukic M, Radonjic T, Opric S, Klasnja S, et al. Lipotoxicity as the leading cause of non-alcoholic steatohepatitis. Int J Mol Sci. 2022;23(9). https://doi.org/10.3390/ijms23095146.
Lusis AJ, Seldin MM, Allayee H, Bennett BJ, Civelek M, Davis RC, et al. The Hybrid Mouse Diversity Panel: a resource for systems genetics analyses of metabolic and cardiovascular traits. J Lipid Res. 2016;57(6):925–42. https://doi.org/10.1194/jlr.R066944.
• Norheim F, Chella Krishnan K, Bjellaas T, Vergnes L, Pan C, Parks BW, et al. Genetic regulation of liver lipids in a mouse model of insulin resistance and hepatic steatosis. Mol Syst Biol. 2021;17(1):e9684. https://doi.org/10.15252/msb.20209684. (This study examined the role of lipid species’ role in NAFLD, using various analytical methods on data from 100 inbred mice strains fed a high-fat/high-sucrose diet. They discovered two genes, Ifi203 and Map2k6, that control phosphatidylcholine homeostasis and triacylglycerol accumulation in the liver, respectively.)
Drozdz K, Nabrdalik K, Kwiendacz H, Hendel M, Olejarz A, Tomasik A, et al. Risk factors for cardiovascular disease in patients with metabolic-associated fatty liver disease: a machine learning approach. Cardiovasc Diabetol. 2022;21(1):240. https://doi.org/10.1186/s12933-022-01672-9.
Duell PB, Welty FK, Miller M, Chait A, Hammond G, Ahmad Z, et al. Nonalcoholic fatty liver disease and cardiovascular risk: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42(6):e168–85. https://doi.org/10.1161/ATV.0000000000000153.
Friedrich-Rust M, Schoelzel F, Maier S, Seeger F, Rey J, Fichtlscherer S, et al. Severity of coronary artery disease is associated with non-alcoholic fatty liver disease: a single-blinded prospective mono-center study. PLoS One. 2017;12(10):e0186720. https://doi.org/10.1371/journal.pone.0186720.
Zhang Z, Zheng M, Lei H, Jiang Z, Chen Y, He H, et al. A clinical study of the correlation between metabolic-associated fatty liver disease and coronary plaque pattern. Sci Rep. 2023;13(1):7224. https://doi.org/10.1038/s41598-023-34462-8.
Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537–64. https://doi.org/10.1016/j.cell.2021.04.015.
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–22. https://doi.org/10.1038/s41591-018-0104-9.
•• Finney AC, Das S, Kumar D, McKinney MP, Cai B, Yurdagul A Jr, et al. The interplay between nonalcoholic fatty liver disease and atherosclerotic cardiovascular disease. Front Cardiovasc Med. 2023;10:1116861. https://doi.org/10.3389/fcvm.2023.1116861. (This review is focused on the intricate connections between nonalcoholic fatty liver disease and cardiovascular disease. It also ventures into the exploration of potential therapeutic strategies targeting both ailments simultaneously)
Chew NWS, Chong B, Ng CH, Kong G, Chin YH, **ao W, et al. The genetic interactions between non-alcoholic fatty liver disease and cardiovascular diseases. Front Genet. 2022;13:971484. https://doi.org/10.3389/fgene.2022.971484.
Diogo D, Tian C, Franklin CS, Alanne-Kinnunen M, March M, Spencer CCA, et al. Phenome-wide association studies across large population cohorts support drug target validation. Nat Commun. 2018;9(1):4285. https://doi.org/10.1038/s41467-018-06540-3.
Ruschenbaum S, Schwarzkopf K, Friedrich-Rust M, Seeger F, Schoelzel F, Martinez Y, et al. Patatin-like phospholipase domain containing 3 variants differentially impact metabolic traits in individuals at high risk for cardiovascular events. Hepatol Commun. 2018;2(7):798–806. https://doi.org/10.1002/hep4.1183.
Wu JT, Liu SS, **e XJ, Liu Q, **n YN, Xuan SY. Independent and joint correlation of PNPLA3 I148M and TM6SF2 E167K variants with the risk of coronary heart disease in patients with non-alcoholic fatty liver disease. Lipids Health Dis. 2020;19(1):29. https://doi.org/10.1186/s12944-020-01207-9.
BasuRay S, Smagris E, Cohen JC, Hobbs HH. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology. 2017;66(4):1111–24. https://doi.org/10.1002/hep.29273.
Linden D, Ahnmark A, **itore P, Ciociola E, Ahlstedt I, Andreasson AC, et al. Pnpla3 silencing with antisense oligonucleotides ameliorates nonalcoholic steatohepatitis and fibrosis in Pnpla3 I148M knock-in mice. Mol Metab. 2019;22:49–61. https://doi.org/10.1016/j.molmet.2019.01.013.
Petta S, Valenti L, Marchesini G, Di Marco V, Licata A, Camma C, et al. PNPLA3 GG genotype and carotid atherosclerosis in patients with non-alcoholic fatty liver disease. PLoS One. 2013;8(9):e74089. https://doi.org/10.1371/journal.pone.0074089.
Musso G, Cassader M, Gambino R. PNPLA3 rs738409 and TM6SF2 rs58542926 gene variants affect renal disease and function in nonalcoholic fatty liver disease. Hepatology. 2015;62(2):658–9. https://doi.org/10.1002/hep.27643.
Acknowledgements
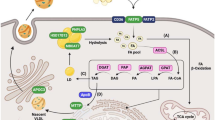
The figure is created with BioRender.com.
Funding
Shirin Pourteymour has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 801133.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pourteymour, S., Drevon, C.A., Dalen, K.T. et al. Mechanisms Behind NAFLD: a System Genetics Perspective. Curr Atheroscler Rep 25, 869–878 (2023). https://doi.org/10.1007/s11883-023-01158-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-023-01158-3