Abstract
Purpose of Review
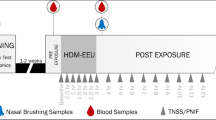
This paper explores how the Environmental Exposure Unit (EEU) experimental model can be used to further our understanding of pharmacotherapies and immunotherapies for the treatment of allergic rhinitis (AR).
Recent Findings
EEUs are used increasingly for the study of combination therapies, immunotherapies, and novel AR treatments. A combined antihistamine/corticosteroid nasal spray formulation was seen to have a faster onset of action relative to the therapies individually in the Environmental Exposure Chamber. House dust mite sublingual immunotherapy tablets are both safe and efficacious as evaluated by the Vienna Challenge Chamber. The Kingston EEU found that a novel peptide-based immunotherapy approach to be effective in reducing grass pollen-induced AR. Lastly, nasal filters were determined to reduce seasonal AR symptoms, given out-of-season in the Denmark Environmental Exposure Unit.
Summary
EEUs are controlled, replicable models that provide valuable insight into the efficacy, onset and duration of action, and dose-related impacts of AR therapeutics, with direct clinical relevance.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lakhani N, North M, Ellis A. Clinical manifestations of allergic rhinitis. J Allergy Ther. 2012;01. https://doi.org/10.4172/2155-6121.S5-007.
Keith PK, Desrosiers M, Laister T, Schellenberg RR, Waserman S. The burden of allergic rhinitis (AR) in Canada: perspectives of physicians and patients. Allergy, Asthma Clin Immunol. 2012;8. https://doi.org/10.1186/1710-1492-8-7.
Meltzer EO, Gross GN, Katial R, Storms WW. Allergic rhinitis substantially impacts patient quality of life: findings from the Nasal Allergy Survey Assessing Limitations. J Fam Pract. 2012;61.
• Passali D, Cingi C, Staffa P, Passali F, Muluk NB, Bellussi ML. The international study of the allergic rhinitis survey: outcomes from 4 geographical regions. Asia Pac Allergy. 2018;8. https://doi.org/10.5415/apallergy.2018.8.e7. This is a cross-sectional, a questionnaire survey concerning AR was completed across different countries in Asia, Europe, the Americas, and Africa. The prevalence of AR was reported to be 15%-25%.
Small P, Keith PK, Kim H. Allergic rhinitis. Allergy, Asthma Clin Immunol. 2018;14:51.
Day JH, Ellis AK, Rafeiro E, Ratz JD, Briscoe MP. Experimental models for the evaluation of treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2006;96:263–78.
Ellis AK, North ML, Walker T, Steacy LM. Environmental exposure unit: a sensitive, specific, and reproducible methodology for allergen challenge. Ann Allergy Asthma Immunol. 2013;111:323–8.
Day JH, Horak F, Briscoe MP, Canonica GW, Fineman SM, Krug N, et al. The role of allergen challenge chambers in the evaluation of anti-allergic medication: an international consensus paper. Clin Exp Allergy Rev. 2006;6:31–59.
•• Ellis AK, Jacobs RL, Tenn MW, et al (2019) Clinical standardization of two controlled allergen challenge facilities: the Environmental Exposure Unit and the Biogenics Research Chamber. Ann Allergy, Asthma Immunol 122:639-646.e2. This article demonstrates equivalent results between 2 CACFs, one located in Kingston, Canada and the other in San Antonio, USA in a double-blind,placebo-controlled, crossover intervention trial.
Horak, F. and SJ (1987) The Vienna challenge chamber (VCC)—a new method for allergen exposition tests. Wiener Klin Wochenschrift 99:509–510.
Pross HF, Day JH, Clark RH, Lees REM. Immunologic studies of subjects with asthma exposed to formaldehyde and urea-formaldehyde foam insulation (UFFI) off products. J Allergy Clin Immunol. 1987;79:797–810.
A pilot study evaluating the signs and symptoms of seasonal allergic rhinoconjunctivitis following exposure in the Allergen BioCube - Full Text View - ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/study/NCT00985075. Accessed 31 Mar 2020.
Ramirez DA, Jacobs RL, Andrews CP. Juniperus asheii (mountain cedar) pollen utilized as an antigen in the biogenics chamber: comparison of natural and controlled exposures. J Allergy Clin Immunol. 2011;127:AB19–9.
Jacobs RL, Ramirez DA, Andrews CP. Validation of the biogenics research chamber for Juniperus ashei (mountain cedar) pollen. Ann Allergy Asthma Immunol. 2011;107:133–8.
Rønborg SM, Mosbech H, Johnsen CR, Poulsen LK. Exposure chamber for allergen challenge the development and validation of a new concept. Allergy. 1996;51:82–8.
Kelly S, Yang J, Perrins R, Karsh J, Yang WH. Technical evaluation of an allergen Challenge Theatre™. Allergy, Asthma Clin Immunol. 2014;10:A22.
Krug N, Loedding H, Hohlfeld JM, Larbig M, Buckendahl A, Badorrek P, et al. Validation of an environmental exposure unit for controlled human inhalation studies with grass pollen in patients with seasonal allergic rhinitis. Clin Exp Allergy. 2003;33:1667–74.
Hamasaki S, Okamoto Y, Yonekura S, Okuma Y, Sakurai T, Iinuma T, et al. Characteristics of the Chiba environmental challenge chamber. Allergol Int. 2014;63:41–50.
Ito K, Terada T, Yuki A, Ichihara T, Hyo S, Kawata R, et al. Preliminary study of a challenge test to the patients with Japanese cedar pollinosis using an environmental exposure unit. Auris Nasus Larynx. 2010;37:694–9.
Baroody FM, Naclerio RM. Antiallergic effects of H1-receptor antagonists. Allergy Eur. J. Allergy Clin. Immunol. Suppl. 2000:17–27.
•• Fein MN, Fischer DA, O’Keefe AW, Sussman GL. CSACI position statement: newer generation H1-antihistamines are safer than first-generation H1-antihistamines and should be the first-line antihistamines for the treatment of allergic rhinitis and urticaria. Allergy, Asthma Clin Immunol. 2019;15:61. A CSACI position statement presenting that newer generation H1-antihistamines are safer than first-generation H1-antihistamines and should be the first-line antihistamines for the treatment of allergic rhinitis and urticaria.
• Kawauchi H, Yanai K, Wang DY, Itahashi K, Okubo K. Antihistamines for allergic rhinitis treatment from the viewpoint of nonsedative properties. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20010213. This article evaluates the non-sedative effects of antihistamines in AR treatment, recommending non-brain-penetrating antihistamines as first-line therapy of mild AR.
Day JH, Briscoe MP, Clark RH, Ellis AK, Gervais P. Onset of action and efficacy of terfenadine, astemizole, cetirizine, and loratadine for the relief of symptoms of allergic rhinitis. Ann Allergy Asthma Immunol. 1997;79:163–72.
Day JH, Briscoe M, Widlitz MD. Cetirizine, loratadine, or placebo in subjects with seasonal allergic rhinitis: effects after controlled ragweed pollen challenge in an environmental exposure unit. J Allergy Clin Immunol. 1998;101:638–45.
Day JH, Briscoe M, Rafeiro E, Chapman D, Kramer B. Comparative onset of action and symptom relief with cetirizine, loratadine, or placebo in an environmental exposure unit in subjects with seasonal allergic rhinitis: confirmation of a test system. Ann Allergy Asthma Immunol. 2001;87:474–81.
• Snidvongs K, Seresirikachorn K, Khattiyawittayakun L, Chitsuthipakorn W. Sedative effects of levocetirizine: a systematic review and meta-analysis of randomized controlled studies. Drugs. 2017;77:175–86. This study investigates the sedative effects of levocetirizine compared to placebo, demonstrating modest effects, though not significantly different fromother second-generation antihistamines.
Tillement J-P, Testa B, Brée F. Compared pharmacological characteristics in humans of racemic cetirizine and levocetirizine, two histamine H1-receptor antagonists. Biochem Pharmacol. 2003;66:1123–6.
Stübner P, Zieglmayer R, Horak F. A direct comparison of the efficacy of antihistamines in SAR and PAR: randomised, placebo-controlled studies with levocetirizine and loratadine using an environmental exposure unit - the Vienna Challenge Chamber (VCC). Curr Med Res Opin. 2004;20:891–902.
Geha RS, Meltzer EO. Desloratadine: a new, nonsedating, oral antihistamine. J Allergy Clin Immunol. 2001;107:751–62.
Day JH, Briscoe MP, Rafeiro E, Ratz JD. Comparative clinical efficacy, onset and duration of action of levocetirizine and desloratadine for symptoms of-seasonal allergic rhinitis in subjects evaluated in the Environmental Exposure Unit (EEU). Int J Clin Pract. 2004;58:109–18.
Horak F, Zieglmayer PU, Zieglmayer R, Kavina A, Lemell P. Levocetirizine has a longer duration of action on improving total nasal symptoms score than fexofenadine after single administration. Br J Clin Pharmacol. 2005;60:24–31.
Terrien MH, Rahm F, Fellrath JM, Spertini F. Comparison of the effects of terfenadine with fexofenadine on nasal provocation tests with allergen. J Allergy Clin Immunol. 1999;103:1025–30.
Day JH, Briscoe MP, Rafeiro E, Hewlett D, Chapman D, Kramer B. Randomized double-blind comparison of cetirizine and fexofenadine after pollen challenge in the Environmental Exposure Unit: duration of effect in subjects with seasonal allergic rhinitis. Allergy Asthma Proc. 2004;25:59–68.
Day JH, Briscoe MP, Rafeiro E, Ratz JD, Ellis AK, Frankish CW, et al. Comparative efficacy of cetirizine and fexofenadine for seasonal allergic rhinitis, 5-12 hours postdose, in the environmental exposure unit. Allergy Asthma Proc. 2005;26:275–82.
Day JH, Briscoe MP, Welsh A, Smith JN, Clark A, Ellis AK, et al. Onset of action, efficacy, and safety of a single dose of fexofenadine hydrochloride for ragweed allergy using an environmental exposure unit. Ann Allergy Asthma Immunol. 1997;79:533–40.
Horak F, Zieglmayer P, Zieglmayer R, Lemell P. The effects of bilastine compared with cetirizine, fexofenadine, and placebo on allergen-induced nasal and ocular symptoms in patients exposed to aeroallergen in the Vienna Challenge Chamber. Inflamm Res. 2010;59:391–8.
Ellis AK, Zhu Y, Steacy LM, Walker T, Day JH. A four-way, double-blind, randomized, placebo controlled study to determine the efficacy and speed of azelastine nasal spray, versus loratadine, and cetirizine in adult subjects with allergen-induced seasonal allergic rhinitis. Allergy, Asthma Clin Immunol. 2013;9. https://doi.org/10.1186/1710-1492-9-16.
•• Tenn MW, Steacy LM, Ng CC, Ellis AK. Onset of action for loratadine tablets for the symptomatic control of seasonal allergic rhinitis in adults challenged with ragweed pollen in the Environmental Exposure Unit: a post hoc analysis of total symptom score. Allergy, Asthma Clin Immunol. 2018;14:5. In this article, the onset of action of loratadine tablets was determined to be 75 mins was investigtaed in the Environmental Exposure Unit for seasonal AR.
Horak F, Zieglmayer UP, Zieglmayer R, Kavina A, Marschall K, Munzel U, et al. Azelastine nasal spray and desloratadine tablets in pollen-induced seasonal allergic rhinitis: a pharmacodynamic study of onset of action and efficacy. Curr Med Res Opin. 2006;22:151–7.
Patel P, Roland PS, Marple BF, Benninger PJ, Margalias H, Brubaker M, et al. An assessment of the onset and duration of action of olopatadine nasal spray. Otolaryngol Head Neck Surg. 2007;137:918–24.
Yonekura S, Okamoto Y, Yamamoto H, Sakurai T, Iinuma T, Sakurai D, et al. Randomized double-blind study of prophylactic treatment with an antihistamine for seasonal allergic rhinitis. Int Arch Allergy Immunol. 2013;162:71–8.
Stuebner P, Horak F, Zieglmayer R, Arnáiz E, Leuratti C, Pérez I, et al. Effects of rupatadine vs placebo on allergen-induced symptoms in patients exposed to aeroallergens in the Vienna Challenge Chamber. Ann Allergy Asthma Immunol. 2006;96:37–44.
Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin Exp Allergy. 2006;36:689–703.
Day JH, Briscoe MP, Ratz JD. Efficacy of levocetirizine compared with montelukast in subjects with ragweed-induced seasonal allergic rhinitis in the environmental exposure unit. Allergy Asthma Proc. 2008;29:304–12.
Patel P, Patel D. Efficacy comparison of levocetirizine vs montelukast in ragweed sensitized patients. Ann Allergy Asthma Immunol. 2008;101:287–94.
Trangsrud AJ, Whitaker AL, Small RE. Intranasal corticosteroids for allergic rhinitis. Pharmacotherapy. 2002;22:1458–67.
•• Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines—2016 revision. J Allergy Clin Immunol. 2017;140:950–8. The Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines present recommendations to support patients, their caregivers, and health care providers in choosing the optimal AR treatment, which might improve patients' quality of life and school and work productivity.
Mabry RL. Corticosteroids in the management of upper respiratory allergy: the emerging role of steroid nasal sprays. Otolaryngol Head Neck Surg. 1992;107:855–9 discussion 859-60.
Day JH, Briscoe MP, Rafeiro E, Ellis AK, Pettersson E, Åkerlund A. Onset of action of intranasal budesonide (Rhinocort aqua) in seasonal allergic rhinitis studied in a controlled exposure model. J Allergy Clin Immunol. 2000;105:489–94.
Day JH, Buckeridge DL, Clark RH, Briscoe MP, Phillips R. A randomized, double-blind, placebo-controlled, controlled antigen delivery study of the onset of action of aerosolized triamcinolone acetonide nasal spray in subjects with ragweed-induced allergic rhinitis. J Allergy Clin Immunol. 1996;97:1050–7.
Study using the Environmental Exposure Unit (EEU) to assess the onset of action of ciclesonide, applied as a nasal spray in the treatment of seasonal allergic rhinitis (BY9010/M1-407) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00659503?term=BY9010%2FM1&cond=Seasonal+Allergic+Rhinitis&draw=2&rank=5. Accessed 7 Apr 2020.
A study of ciclesonide nasal spray in patients 18 years and older with seasonal allergic rhinitis (BY9010/M1-413) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00384475?term=M1&cond=allergic+rhinitis&draw=2&rank=10. Accessed 15 Apr 2020.
Badorrek P, Hohlfeld JM, Krug N, Joshi A, Raut A. Efficacy and safety of a novel nasal steroid, S0597, in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2015;115:325–329.e1.
Ellis AK, Steacy LM, Joshi A, Bhowmik S, Raut A. Efficacy of the novel nasal steroid S0597 tested in an environmental exposure unit. Ann Allergy Asthma Immunol. 2016;117:310–7.
Patel P, D’Andrea C, Sacks HJ. Onset of action of azelastine nasal spray compared with mometasone nasal spray and placebo in subjects with seasonal allergic rhinitis evaluated in an environmental exposure chamber. Am J Rhinol. 2007;21:499–503.
Patel D, Garadi R, Brubaker M, Conroy PJ, Kaji Y, Crenshaw K, et al. Onset and duration of action of nasal sprays in seasonal allergic rhinitis patients: Olopatadine hydrochloride versus mometasone furoate monohydrate. Allergy Asthma Proc. 2007;28:592–9.
Van Cauwenberge P, Van Hcecke H, Bousquet J (2005) Allergic rhinitis and its impact on asthma. In: Pediatr. Nasal Sinus Disord. CRC Press, pp 402–422.
Yamamoto H, Yonekura S, Sakurai D, Katada K, Inamine A, Hanazawa T, et al. Comparison of nasal steroid with antihistamine in prophylactic treatment against pollinosis using an environmental challenge chamber. Allergy Asthma Proc. 2012;33:397–403.
Zieglmayer P, Zieglmayer R, Bareille P, Rousell V, Salmon E, Horak F. Fluticasone furoate versus placebo in symptoms of grass-pollen allergic rhinitis induced by exposure in the Vienna Challenge Chamber. Curr Med Res Opin. 2008;24:1833–40.
Salapatek AM, Patel P, Gopalan G, Varghese ST. Mometasone furoate nasal spray provides early, continuing relief of nasal congestion and improves nasal patency in allergic patients. Am J Rhinol Allergy. 2010;24:433–8.
Day JH, Briscoe MP, Ratz JD, Ellis AK, Yao R, Danzig M. Onset of action of loratadine/montelukast in seasonal allergic rhinitis subjects exposed to ragweed pollen in the Environmental Exposure Unit. Allergy Asthma Proc. 2009;30:270–6.
Horak F, Zieglmayer P, Zieglmayer R, Lemell P. Onset of action of loratadine/montelukast in seasonal allergic rhinitis patients exposed to grass pollen. Arzneimittel-Forschung/Drug Res. 2010;60:249–55.
Johnson DA, Hricik JG. The pharmacology of α-adrenergic decongestants. Pharmacother J Hum Pharmacol Drug Ther. 1993;13:110S–5S.
Badorrek P, Dick M, Schauerte A, Hecker H, Murdoch R, Luettig B, et al. A combination of cetirizine and pseudoephedrine has therapeutic benefits when compared to single drug treatment in allergic rhinitis. Int J Clin Pharmacol Ther. 2009;47:71–7.
Zieglmayer UP, Horak F, Toth J, Marks B, Berger UE, Burtin B. Efficacy and safety of an oral formulation of cetirizine and prolonged-release pseudoephedrine versus budesonide nasal spray in the management of nasal congestion in allergic rhinitis. Treat Respir Med. 2005;4:283–7.
Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Yao R, Staudinger H, et al. A placebo-controlled study of the nasal decongestant effect of phenylephrine and pseudoephedrine in the Vienna Challenge Chamber. Ann Allergy Asthma Immunol. 2009;102:116–20.
Day JH, Briscoe MP, Ratz JD, Danzig M, Yao R. Efficacy of loratadine-montelukast on nasal congestion in patients with seasonal allergic rhinitis in an environmental exposure unit. Ann Allergy Asthma Immunol. 2009;102:328–38.
Berkowitz RB, Woodworth GG, Lutz C, Weiler K, Weiler J, Moss M, et al. Onset of action, efficacy, and safety of fexofenadine 60 mg/pseudoephedrine 120 mg versus placebo in the Atlanta allergen exposure unit. Ann Allergy Asthma Immunol. 2002;89:38–45.
Chervinsky P, Nayak A, Rooklin A, Danzig M. Efficacy and safety of desloratadine/pseudoephedrine tablet, 2.5/120 mg two times a day, versus individual components in the treatment of patients with seasonal allergic rhinitis. Allergy asthma Proc. 2005;26:391–6.
Daley-Yates P, Ambery C, Sweeney L, Watson J, Oliver A, McQuade B. The efficacy and tolerability of two novel H 1/H 3 receptor antagonists in seasonal allergic rhinitis. Int Arch Allergy Immunol. 2012;158:84–98.
•• North ML, Walker T, Steacy LM, Hobsbawn BG, Allan RJ, Hackman F, et al. Double blind randomized crossover trial of PF-03654764+fexofenadine in the environmental exposure unit (EEU). Allergy, Asthma Clin Immunol. 2014;10:A68. This double blind randomized crossover trial of PF-03654764+fexofenadine in the Environmental Exposure Unit found improved TNSS compared toplacebo, with insignificant side effects.
•• Roca-Ferrer J, Pujols L, Pérez-González M, Alobid I, Callejas B, Vicens-Artés S, et al. Superior effect of MP-AzeFlu than azelastine or fluticasone propionate alone on reducing inflammatory markers. Allergy, Asthma Clin Immunol. 2018;14:86. This study found superior clinical efficacy with MP-AzeFlu than azelastine or fluticasone propionate alone in reducing inflammatory markers.
Bousquet J, Meltzer EO, Couroux P, Koltun A, Kopietz F, Munzel U, et al. Onset of action of the fixed combination intranasal azelastine-fluticasone propionate in an allergen exposure chamber. J Allergy Clin Immunol Pract. 2018;6:1726–1732.e6.
Salapatek AM, Lee J, Patel D, D’Angelo P, Liu J, Zimmerer RO, et al. Solubilized nasal steroid (CDX-947) when combined in the same solution nasal spray with an antihistamine (CDX-313) provides improved, fast-acting symptom relief in patients with allergic rhinitis. Allergy Asthma Proc. 2011;32:221–9.
Moote W, Kim H. Allergen-specific immunotherapy. Allergy, Asthma Clin Immunol. 2011;7:S5.
• Donovan JP, Buckeridge DL, Briscoe MP, Clark RH, Day JH. Efficacy of immunotherapy to ragweed antigen tested by controlled antigen exposure. Ann Allergy Asthma Immunol. 1996;77:74–80. Ragweed antigen immunotherapy was evaluated in a controlled ragweed allergen exposure showed significantly reduced symptoms of ragweed-allergic rhinitis with no significant effect on ocular symptoms.
Roux M, Viatte A, Zeldin RK. Safety of a sublingual tablet of house dust mite allergen extracts in an environmental exposure chamber study. J Allergy Clin Immunol. 2015;135:AB266.
Roux M, Yang WH, Viatte A, Cadic VZR. Efficacy of house dust mite sublingual tablets in an environmental exposure chamber study of patients with house dust mite-associated allergic rhinitis | Cochrane Library. Allergy Eur J allergy Clin Immunol. 2013;68:5–6.
Nolte H, Maloney J, Nelson HS, et al. Onset and dose-related efficacy of house dust mite sublingual immunotherapy tablets in an environmental exposure chamber. J Allergy Clin Immunol. 2015;135:1494–1501.e6.
Horak F, Zieglmayer P, Zieglmayer R, et al. Early onset of action of a 5-grass-pollen 300-IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol. 2009;124:471–7 477.e1.
Ellis AK, Tenn MW, Steacy LM, Adams DE, Day AG, Walker TJ, et al. Lack of effect of Timothy grass pollen sublingual immunotherapy tablet on birch pollen–induced allergic rhinoconjunctivitis in an environmental exposure unit. Ann Allergy, Asthma Immunol. 2018;120:495–503.e2.
Ellis AK, Frankish CW, O’Hehir RE, Armstrong K, Steacy L, Larché M, et al. Treatment with grass allergen peptides improves symptoms of grass pollen–induced allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2017;140:486–96.
•• Patel P, Holdich T, Fischer Von Weikersthal-Drachenberg KJ, Huber B (2014) Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J Allergy Clin Immunol 133:121–9.e1–2. This study demonstrated that an ultrashort course of ragweed immunotherapy is efficacious in reducing allergy symptoms in patients with seasonal AR and that it is well tolerated.
Patel D, Couroux P, Hickey P, Salapatek AM, Laidler P, Larché M, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131:103–9.e1–7.
Horak F, Zieglmayer P, Zieglmayer R, et al. The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial. Allergy Eur J Allergy Clin Immunol. 2012;67:1572–9.
Krug N, Gupta A, Badorrek P, Koenen R, Mueller M, Pivovarova A, et al. Efficacy of the oral chemoattractant receptor homologous molecule on T H2 cells antagonist BI 671800 in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 2014;133:414–9.
Kenney P, Hilberg O, Pedersen H, Nielsen OB, Sigsgaard T. Rhinix™ nasal filters for the treatment of allergic rhinitis: a randomized, double-blinded placebo-controlled crossover clinical trial. J Allergy Clin Immunol. 2014;133:AB186.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Ellis has participated in advisory boards for ALK Abello, AstraZeneca, Aralez, Bausch Health, Circassia Ltd., GlaxoSmithKline, Johnson & Johnson, Merck, Mylan, Novartis, Pediapharm, and Pfizer, has been a speaker for ALK, Aralez, AstraZeneca, Boerhinger-Ingleheim, CACME, Meda, Mylan, Merck, Novartis, Pediapharm, Pfizer, The ACADEMY, and Takeda. Her institution has received research grants from Bayer LLC, Circassia Ltd., Green Cross Pharmaceuticals, GlaxoSmithKline, Sun Pharma, Merck, Novartis, Pfizer, Regeneron, and Sanofi. She has also served as an independent consultant to Allergy Therapeutics, Bayer LLC, Ora Inc., and Regeneron in the past. The other authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Rhinitis, Conjunctivitis, and Sinusitis
Rights and permissions
About this article
Cite this article
Hossenbaccus, L., Steacy, L.M., Walker, T. et al. Utility of Environmental Exposure Unit Challenge Protocols for the Study of Allergic Rhinitis Therapies. Curr Allergy Asthma Rep 20, 34 (2020). https://doi.org/10.1007/s11882-020-00922-8
Published:
DOI: https://doi.org/10.1007/s11882-020-00922-8