Abstract
Background
Dry eye is a condition related to long-term topical eye therapy. We wish to evaluate the effectiveness and tolerability of preservative free prostaglandin drops versus benzalkonium chloride containing prostaglandin drops in the treatment of glaucoma.
Methods
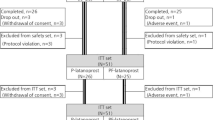
Patients undergoing prostaglandin monotherapy underwent a washout period of at least 1 month after which baseline measurements of dry eye severity were taken. Patients were randomised to receive either 0.0015% tafluprost drops or 0.005% latanoprost preserved with 0.02% benzalkonium chloride. Repeat measurements were taken after a 2-month interval.
Results
Thirty-five patients completed randomised treatment. No significant difference between groups was found in objective and subjective measurements of dry eye severity. No significant difference was found in measurement of treatment effectiveness.
Conclusion
Preservative-free and benzalkonium chloride–containing drops were found to be equally effective in lowering IOP with no significant difference in either subjective or objective measurements of dry eye severity.

Similar content being viewed by others
Data availability
The data that support this study are not openly available but are available from the corresponding author upon reasonable request.
References
Tham YC, Li X, Wong TY et al (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121(11):2081–2090
Baudouin C, Denoyer A, Desbenoit N et al (2012) In vitro and in vivo experimental studies on trabecular meshwork degeneration induced by benzalkonium chloride (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 110:40–63
Brignole-Baudouin F, Desbenoit N, Hamm G et al (2012) A new safety concern for glaucoma treatment demonstrated by mass spectrometry imaging of benzalkonium chloride distribution in the eye, an experimental study in rabbits. PLoS ONE 7(11)
Lee S, Kim MK, Choi HJ et al (2013) Comparative cross-sectional analysis of the effects of topical antiglaucoma drugs on the ocular surface. Adv Ther 30(4):420–429
Leung EW, Medeiros FA, Weinreb RN (2008) Prevalence of ocular surface disease in glaucoma patients. J Glaucoma 17(5):350–355
Okeke CO, Quigley HA, Jampel HD et al (2009) Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology 116(2):191–199
Steven DW, Alaghband P, Lim KS (2018) Preservatives in glaucoma medication. Br J Ophthalmol 102(11):1497–1503
Hedengran A, Steensberg AT, Virgili G et al (2020) Efficacy and safety evaluation of benzalkonium chloride preserved eye-drops compared with alternatively preserved and preservative-free eye-drops in the treatment of glaucoma: a systematic review and meta-analysis. Br J Ophthalmol 104(11):1512–1518
(2007) The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop (2007). Ocul Surf 5(2):75–92. https://doi.org/10.1016/s1542-0124(12)70081-2
Farris RL (1994) Tear osmolarity–a new gold standard? Adv Exp Med Biol 350:495–503
Wolffsohn JS, Arita R, Chalmers R et al (2017) TFOS DEWS II Diagnostic Methodology report. Ocul Surf 15(3):539–574
Nichols KK, Mitchell GL, Zadnik K (2004) The repeatability of clinical measurements of dry eye. Cornea 23(3):272–285
Lemp MA (1995) Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J 21(4):221–232
Vitali C, Bombardieri S, Jonsson R et al (2002) Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61(6):554–558
TearLab Inc (2016) DEWS Calculator
Economou MA, Laukeland HK, Grabska-Liberek I et al (2018) Better tolerance of preservative-free latanoprost compared to preserved glaucoma eye drops: the 12-month real-life FREE study. Clin Ophthalmol 12:2399–2407
Rouland JF, Traverso CE, Stalmans I et al (2013) Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br J Ophthalmol 97(2):196–200
Hommer A, Schmidl D, Kromus M et al (2018) Effect of changing from preserved prostaglandins to preservative-free tafluprost in patients with glaucoma on tear film thickness. Eur J Ophthalmol 28(4):385–392
Janulevičienė I, Derkač I, Grybauskiene L et al (2012) Effects of preservative-free tafluprost on tear film osmolarity, tolerability, and intraocular pressure in previously treated patients with open-angle glaucoma. Clin Ophthalmol (Auckland, NZ) 6:103–109
Hamacher T, Airaksinen J, Saarela V et al (2008) Efficacy and safety levels of preserved and preservative-free tafluprost are equivalent in patients with glaucoma or ocular hypertension: results from a pharmacodynamics analysis. Acta Ophthalmol Suppl (Oxf) 242:14–19
Mizoguchi T, Ozaki M, Unoki K et al (2012) A randomized crossover study comparing tafluprost 0.0015% with travoprost 0.004% in patients with normal-tension glaucoma [corrected]. Clin Ophthalmol 6:1579–1584
Uusitalo H, Pillunat LE, Ropo A (2010) Efficacy and safety of tafluprost 0.0015% versus latanoprost 0.005% eye drops in open-angle glaucoma and ocular hypertension: 24-month results of a randomized, double-masked phase III study. Acta Ophthalmol 88(1):12–9
Hommer A, Mohammed Ramez O, Burchert M et al (2010) IOP-lowering efficacy and tolerability of preservative-free tafluprost 0.0015% among patients with ocular hypertension or glaucoma. Curr Med Res Opin 26(8):1905–13
Uusitalo H, Chen E, Pfeiffer N et al (2010) Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication. Acta Ophthalmol 88(3):329–336
Walimbe T, Chelerkar V, Bhagat P et al (2016) Effect of benzalkonium chloride-free latanoprost ophthalmic solution on ocular surface in patients with glaucoma. Clin Ophthalmol 10:821–827
Katz G, Springs CL, Craven ER et al (2010) Ocular surface disease in patients with glaucoma or ocular hypertension treated with either BAK-preserved latanoprost or BAK-free travoprost. Clin Ophthalmol (Auckland, NZ) 4:1253–1261
Labbe A, Terry O, Brasnu E et al (2012) Tear film osmolarity in patients treated for glaucoma or ocular hypertension. Cornea 31(9):994–999
Gómez-Aguayo F, Paczka JA, Leñero-Córdova R et al (2018) A phase III randomized clinical trial of a 0.5% Timolol + 0.2% Brimonidine + 2.0% Dorzolamide fixed combination, preservative-free ophthalmic solution vs. 0.5% Timolol + 0.2% Brimonidine + 2.0% Dorzolamide fixed combination in patients with controlled primary open-angle glaucoma. Ophthalmol Therapy 7(1):145–56
Lemp MA, Bron AJ, Baudouin C et al (2011) Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 151(5):792–8 e1
Inoue K (2014) Managing adverse effects of glaucoma medications. Clin Ophthalmol (Auckland, NZ) 8:903–913
Halkiadakis I, Kontadakis GA, Tsiakou D et al (2015) Effect of glaucoma medication in tear film osmolarity of patients without symptoms of ocular discomfort. J Ocul Pharmacol Ther 31(6):330–334
El Hajj Moussa WG, Farhat RG, Nehme JC et al (2018) Comparison of efficacy and ocular surface disease index score between bimatoprost, latanoprost, travoprost, and tafluprost in glaucoma patients. J Ophthalmol 2018:1319628
Misiuk-Hojlo M, Pomorska M, Mulak M et al (2018) The RELIEF study: tolerability and efficacy of preservative-free latanoprost in the treatment of glaucoma or ocular hypertension. Eur J Ophthalmol 2018:1120672118785280
Tokuda N, Kitaoka Y, Matsuzawa A et al (2017) Changes in ocular surface characteristics after switching from benzalkonium chloride-preserved latanoprost to preservative-free tafluprost or benzalkonium chloride-preserved tafluprost. J Ophthalmol 2017:3540749
Aihara M, Otani S, Kozaki J et al (2012) Long-term effect of BAK-free travoprost on ocular surface and intraocular pressure in glaucoma patients after transition from latanoprost. J Glaucoma 21(1):60–64
Acknowledgements
The authors would like to thank the participants and the hospital for their resources given willingly and freely, be it time, space, equipment or advice.
Funding
The authors and department received no funding support for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brinkman, D., McSwiney, T. & James, M. Comparing the tolerability of preservative-free tafluprost versus preserved latanoprost in the management of glaucoma and ocular hypertension — an observer blinded active-control trial. Ir J Med Sci (2024). https://doi.org/10.1007/s11845-024-03704-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11845-024-03704-7