Abstract
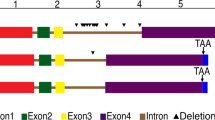
Auxin-binding protein 57 (ABP57), a soluble auxin-binding protein, acts as a receptor to activate plasma membrane (PM) H+-ATPase. Here, we report the cloning of abp57 and the biochemical characterization of its protein expressed in E. coli. The analysis of internal amino acid sequences of ABP57 purified from rice shoots enabled us to search for the corresponding gene in protein DB of NCBI. Further BLAST analysis showed that rice has four abp57-like genes and maize has at least one homolog. Interestingly, Arabidopsis seems to have no homolog. Recombinant ABP57 expressed in E. coli caused the activation of PM H+-ATPase regardless of the existence of IAA. Scatchard analysis showed that the recombinant protein has relatively low affinity to IAA as compared to natural ABP57. These results collectively support the notion that the cloned gene is responsible for ABP57.






Similar content being viewed by others
References
Badescu GO, Napier RM (2006) Receptors for auxin: will it all end in TIRs? Trends Plant Sci 11:217–223
Barbier-Brygoo H, Ephritikhine G, Klambt D, Ghislain M, Guern J (1989) Functional evidence for an auxin receptor at the plasmalemma of tobacco mesophyll protoplasts. Proc Natl Acad Sci USA 86:891–895
Birnbaumer L, Pohl SL, Kaumann AJ (1974) Receptors and acceptors: a necessary distinction in hormone binding studies. In: Greengard P, Robison GG (eds) Advances in cyclic nucleotide research. North-Holland, New York, pp 239–281
Chen JG, Ullah H, Young JC, Sussman MR, Jones AM (2001) ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev 15:902–911
David KM, Couch D, Braun N, Brown S, Grosclaude J, Perrot-Rechenmann C (2007) The auxin-binding protein 1 is essential for the control of cell cycle. Plant J 50:197–206
Dharmasiri N, Dharmasiri S, Estelle M (2005) The F-box protein TIR1 is an auxin receptor. Nature 435:441–445
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hertel R, Thomson KS, Russo VEA (1972) In vitro auxin binding to particulate cell fractions from corn coleoptiles. Planta 107:325–340
Inohara N, Shimomura S, Fukui T, Futai M (1989) Auxin-binding protein located in the endoplasmic reticulum of maize shoots: molecular cloning and complete primary structure. Proc Natl Acad Sci USA 86:3564–3568
Jones AM, Im KH, Savka MA, Wu MJ, DeWitt NG, Shillito R, Binns AN (1998) Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science 282:1114–1117
Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435:446–451
Kim D, Kim YS, Jung J (1997) Involvement of soluble proteinous factors in auxin-induced modulation of P-type ATPase in rice (Oryza sativa L.) seedlings. FEBS Lett 409:273–276
Kim YS, Kim DH, Jung J (1998) Isolation of a novel auxin receptor from soluble fractions of rice (Oryza sativa L.) shoots. FEBS Lett 438:241–244
Kim YS, Kim D, Jung J (2000) Two isoforms of soluble auxin receptor in rice (Oryza sativa L.) plants: binding property for auxin and interaction with plasma membrane H+-ATPase. Plant Growth Regul 32:143–150
Kim YS, Min JK, Kim D, Jung J (2001) A soluble auxin-binding protein, ABP57-purification with anti-bovine serum albumin antibody and characterization of its mechanistic role in the auxin effect on plant plasma membrane H+-ATPase. J Biol Chem 276:10730–10736
Larsson C, Widell S, Kjellbom P, Lester P, Roland D (1987) Preparation of high-purity plasma membranes. Methods Enzymol 148:558–568
Lobler M, Klambt D (1985) Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). I. Purification by immunological methods and characterization. J Biol Chem 260:9848–9853
Napier RM, David KM, Perrot-Rechenmann C (2002) A short history of auxin-binding proteins. Plant Mol Biol 49:339–348
Palmgren MG (1990) An H+-ATPase assay: proton pum** and ATPase activity determined simultaneously in the same sample. Plant Physiol 94:882–886
Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M (1998) The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast grr1p. Genes Dev 12:198–207
Taiz L, Zeiger E (2002) Plant physiology, 3rd edn. Sinauer Associates, Sunderland
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tillmann U, Viola G, Kayser B, Siemeister G, Hesse T, Palme K, Lobler M, Klambt D (1989) cDNA clones of the auxin-binding protein from corn coleoptiles (Zea mays L.)—isolation and characterization by immunological methods. EMBO J 8:2463–2467
Timpte C (2001) Auxin binding protein: curiouser and curiouser. Trends Plant Sci 6:586–590
Woo EJ, Marshall J, Bauly J, Chen JG, Venis M, Napier RM, Pickersgill RW (2002) Crystal structure of auxin-binding protein 1 in complex with auxin. EMBO J 21:2877–2885
Woodward AW, Bartel B (2005) A receptor for auxin. Plant Cell 17:2425–2429
Acknowledgments
This work was supported by the National Academy of Agricultural Science (200901FHT020711430) and the Biogreen 21 Program (2005030103441901) of the Rural Development Administration.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, K., Kim, MI., Kwon, YJ. et al. Cloning and characterization of a gene encoding ABP57, a soluble auxin-binding protein. Plant Biotechnol Rep 3, 293–299 (2009). https://doi.org/10.1007/s11816-009-0101-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-009-0101-z