Abstract
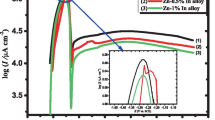
The passivation of pure zinc surface can be considered a problem of Zn utilization as an anode in alkaline batteries due to its small capacity. Therefore, to improve the discharge capacity of the Zn anode, minor Sb alloying with Zn was investigated. The impact of trace Sb alloyed with Zn on the passivity and the breakdown of the colloidal passive film on the surface was studied in concentrated KOH solution utilizing galvanostatic, electrochemical impedance spectroscopy (EIS), and galvanostatic charge-discharge measurements at the passivated area. The galvanostatic data show that the required time of passivation (tpass.) is greater with increasing small Sb content in the alloy. The obtained results from electrochemical impedance spectroscopy (EIS) reveal that magnitudes of both resistivities of charge transfer (Rct.) and the impedance of Warburg (Zw) decrease, while the magnitude of capacitance of double layer (Cdl.) increases gradually with the increase in addition of small Sb to zinc metal. The evaluated data from the charging-discharging process show that the greatest value of potential height (ΔV) is for Zn-0.5%Sb alloy. Therefore, 0.5%Sb alloying with Zn can increase energy efficiency to a large extent than Zn and alloy II.
Similar content being viewed by others
References
L. Yanguang and D. Hongjie, Chem. Soc. Rev., 43, 5257 (2014).
J. S. Lee, S. T. Kim, R. Cao, N. S. Choi, M. Liu, K. T. Lee and J. Cho, Adv. Energy Mater., 1, 34 (2011).
H. Ma and B. Wang, RSC Adv., 4, 46084 (2014).
M. Prabu, P. Ramakrishnan and S. Shahmugam, Electrochem. Commun., 41, 59 (2014).
G. L. Tian, M. Q. Zhao, D. Yu, X. Y. Kong, J. Q. Huang, Q. Zhang and F. Wei, Small, 11, 2251 (2014).
Z. Shao, W. Zhang, D. An, G. Zhang and Y. Wang, RSC Adv., 5, 97508 (2015).
T. M. Bawazeer, A. M. El Defrawy and A. A. El-Shafei, Colloids Surf. A, 520, 694 (2017).
A. Singh, K. R. Ansari and M. A. Quraishi, Colloids Surf. A, 607, 125465 (2020).
J. Huang and Z. Yang, RSC Adv., 5, 33814 (2015).
J. Drillet, M. Adam, S. Barg, A. Herter, D. Koch, V Schmidt and M. Wilhelm, ECS Trans., 28, 13 (2010).
P. Bonnick and J. Dahn, J. Electrochem. Soc., 159, A981 (2012).
W. Hong, Z. Jia and B. Wang, J. Appl. Electrochem., 46, 1085 (2016).
Y. Tian, Y. An, C. Liu, S. **ong, J. Feng and Y. Qian, Energy Storage. Mater., 41, 343 (2021).
C. Zhang, J. M. Wang, L. Zhang, J. Q. Zhang and C. N. Cao, J. Appl. Electrochem., 31, 1049 (2001).
Y. Yu, Y. Zuo, Z. Zhang, L. Wu, C. Ning and C. Zuo, Coatings, 9, 692 (2019).
C. W. Lee K. Sathiyanarayanan, S. W. Eom and M. S. Yun, J. Power Sources, 160, 1436 (2006).
Y. N. Jo, K. Prasanna, S. H. Kang, P. R. IIango, H. S. Kim, W. S. Eom and C. W. Lee, J. Ind. Eng. Chem., 53, 247 (2017).
W. Gan, D. Zhou, L. Zhou, Z. Zhang and J. Zhao, Electrochim. Acta, 182, 430 (2015).
M. Hilder, B. W. Jensen and N. Clark, Electrochim. Acta, 69, 308 (2012).
R. M. Wittman, R. L. Sacci and T. A. Zawodzinski, J. Power Sources, 438, 227034 (2019).
J. Stamm, A. Varzi, A. Latz and B. Horstmann, J. Power Sources, 360, 136 (2017).
M. Elrouby, H. A. S. Shilkamy and A. Elsayed, J. Alloys Compd., 854, 157285 (2021).
A. Elsayed, H. A. S. Shilkamy and M. Elrouby, J. Solid State Electrochem., 25, 2161 (2021).
M. Elrouby, H. A. E. Shilkamy and A. E. R. Elsayed, J. Solid State Electrochem., 25, 2175 (2021).
A. ElSayed, H. A. S. Shilkamy and M. Elrouby, Int. J. Hydrogen Energy, 46, 31239 (2021).
A. ElSayed, A. M. Sahker and H. M. Abd El Lateef, Corrosion Sci., 52, 72 (2010).
A. ElSayed, H. S. Mohran and H. M. Abd El Lateef, Corrosion Sci., 52, 1976 (2010).
H. S. Mohran, A. ElSayed and H. M. Abd El Lateef, Solid State Electrochem., 13, 1147 (2009).
A. ElSayed, A. M. Shaker and H. G. ElKareem, Bull. Chem. Soc. Jpn., 76, 1527 (2003).
H. M. Abd El Lateef, K. Shalabi, A. R. Sayed, S.M. Gomha and E. M. Bakir, J. Ind. Eng. Chem., 105, 238 (2022).
E. E. Abdel Aal, Corrosion Sci., 45, 641 (2003).
E. E. A. El-Aal, Corrosion, 55, 582 (1999).
M. Bockelmann, L. Reining, U. Kunz and T. Turekab, Electrochim. Acta, 237, 276 (2017).
A. Chiba, S. Tanaka, W. Inami, A. Sugita, K. Takada and Y. Kawata, Opt. Mater., 35, 1887 (2013).
E. E. Abd El-Aal, Corrosion Sci., 45, 759 (2003).
D. Gileket, A. Brzózka, K. E. Hnida and G. D. Sulka, Electrochim. Acta, 302, 352 (2019).
A. ElSayed, H. S. Mohran and H. M. Abd El Lateef, Corrosion Sci., 51, 2675 (2009).
A. R. El-Sayed, H. S. Mohran and H. M. Abd El-Lateef, J. Solid State Electrochem., 13, 1279 (2009).
E. Bayol, A. A. Gurten, M. Dursun and K. Kayakirilman, Acta Phys. Chim. Sin., 24, 2236 (2008).
H. M. A. ElLateef, L. I. Aliyeva, V. M. Abbasov and T. I. Ismayilov, Adv. Appl. Sci. Res., 3, 1185 (2012).
H. M. A. EL-Lateef, A. R. ELSayed and H. S. Mohran, J. Trans. Nonferrous Met. Soc. China, 25, 3152 (2015).
E. E. A. El-Aal, Corrosion Sci., 50, 41 (2008).
A. R. El-Sayed and H. M. El-Lateef, Bull. Mater. Sci., 38, 1 (2015).
A. O. Alnajjar, H. M. Abd El Lateef, M. M. Khalaf and I. M. A. Mohammed, Constr. Build. Mater., 317, 25918 (2022).
X. Zeng, Z. Yang, J. Long, L. Chen, H. Qin and M. Fan, Ionics, 25, 1223 (2019).
A. R. El-Sayed, H. S. Mohran and H. M. A. El-Lateef, J. Power Sources, 196, 6573 (2011).
L. Wang, Y. Liu, X. Chen and Z. Yang, Electrochem. Soc., 164, A3692 (2017).
J. He, Y. Wei, T. Zhai and H. Li, Mater. Chem. Front., 2, 437 (2018).
A. H. Abdalla, C. I. Oseghale, J. O. G. Posada and P. J. Hall, IET Renew. Power Gener., 10, 1529 (2016).
S. S. A. El-Rehim, H. H. Hassan and A. Mohammed, Appl. Surf. Sci., 187, 279 (2002).
M. Mouanga and P. Berçot, Corrosion Sci., 52, 3993 (2010).
P. Gu, M. Zheng, Q. Zhao, X. **ao, H. Xue and H. Pang, J. Mater. Chem. A, 5, 7651 (2017).
X. Chen, L. Wang, H. Qin and Z. Yang, Ionics, 25, 1715 (2019).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interest
The authors declared that they have no conflict of interest
Availability of Data And Materials
All data generated or analysed during this study are included in this article.
Rights and permissions
About this article
Cite this article
Elrouby, M., Shilkamy, H.A.ES. & El-Sayed, A.ER. Breakdown of passivation for zinc-antimony alloy in alkaline batteries verification; galvanostatic, impedance spectra, and charge-discharge techniques. Korean J. Chem. Eng. 40, 572–583 (2023). https://doi.org/10.1007/s11814-022-1353-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-022-1353-3