Abstract
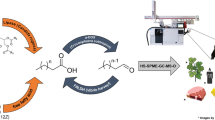
Polyunsaturated long-chain alkenones are a unique class of lipids biosynthesized in significant quantities (up to 20% of cell carbon) by several algae including the industrially grown marine microalgae Isochrysis. Alkenone structures are characterized by a long linear carbon chain (35–40 carbons) with one to four trans double bonds and terminating in a methyl or ethyl ketone. Alkenones were extracted and isolated from commercially obtained Isochrysis biomass and then subjected to cross-metathesis (CM) with methyl acrylate or acrylic acid using the Hoveyda–Grubbs metathesis initiator. Within 1 h at room temperature alkenones were consumed; however, complete fragmentation (i.e., conversion to the smallest subunits by double bond cleavage) required up to 16 h. Analysis of the reaction mixture by gas chromatography and comprehensive two-dimensional gas chromatography revealed a predictable product mixture consisting primarily of long-chain (mostly C17) acids (or methyl esters from CM with methyl acrylate) and diacids (or diesters), along with smaller amounts (~5%) of the honey bee “queen substance” (E)-9-oxo-decenoic acid. Together, these compounds comprise a diverse mixture of valuable chemicals that includes surfactants, monomers, and an agriculturally relevant bee pheromone.







Similar content being viewed by others
References
Solomon S, Daniel JS, Sanford TJ, Murphy DM, Plattner G-K, Knutti R, Friedlingstein P (2010) Persistence of climate changes due to a range of greenhouse gases. Proc Natl Acad Sci 107:18354–18359
Ragauskas J, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ Jr, Hallett JP, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski T (2006) The path forward for biofuels and biomaterials. Science 311:484–489
Christensen CH, Rass-Hansen J, Marsden CC, Taarning E, Egeblad K (2008) The renewable chemicals industry. ChemSusChem 1:283–289
Inderwildi OR, King DA (2009) Quo vadis biofuels. Energy Environ Sci 2:343–346
Corma A, Iborra S, Velty A (2007) Chemical routes for the transformation of biomass into chemicals. Chem Rev 107:2411–2502
Vennestrøm PNR, Osmundsen CM, Christensen CH, Taarning E (2011) Beyond petrochemicals: the renewable chemicals industry. Angew Chem Int Ed 50:10502–10509
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Borowitzka MA (1986) Microalgae as sources of fine chemicals. Microbio Sci 12:372–375
Volkman JK, Eglinton G, Corner EDS (1980) Long-chain alkenes and alkenones in the marine coccolithophorid Emiliania huxleyi. Phytochem 19:2619–2622
Epstein BL, D’Hondt S, Quinn JG, Zhang J, Hargraves P (1998) An effect of dissolved nutrient concentrations on alkenone-based temperature estimates. Paleoceanography 13:122–126
Prahl FG, Wolfe GV, Sparrow MA (2003) Physiological impacts on alkenone paleothermometry. Paleoceanogaphy 18:1025–1031
Eltgroth ML, Watwood RL, Wolfe GV (2005) Production and cellular localization of neutral long-chain lipids in the haptophyte algae Isochrysis Galbana and Emiliania Huxleyi. J Phycol 41:1000–1009
Volkman JK, Everitt DA, Allen DI (1986) Some analyses of lipid classes in marine organisms, sediments and seawater using thin-layer chromatography-flame ionisation detection. J Chromatogr A 356:47–162
Isochrysis can be purchased from Reed Mariculture (2016) (San Jose, CA) at: http://reedmariculture.com and Necton SA (Olhão, Portugal) at: http://www.phytobloom.com. Accessed 11 Nov 2016
O’Neil GW, Knothe G, Williams JR, Burlow NP, Culler AR, Corliss JM, Carmichael CA, Reddy CM (2014) Synthesis and analysis of an alkenone-free biodiesel from Isochrysis sp. Energy Fuels 28:2677–2683
Liu H, Cheng T, **an M, Cao Y, Fang F, Zou H (2014) Fatty acid from the renewable sources: a promising feedstock for the production of biofuels and biobased chemicals. Biotechnol Adv 32:382–389
Warwel S, Brüse F, Demes C, Kunz M, Rusch gen Klaas MR (2001) Polymers and surfactants on the basis of renewable resources. Chemosphere 43:39–48
O’Neil GW, Culler AR, Williams JR, Burlow NP, Gilbert GJ, Carmichael CA, Nelson RK, Swarthout RF, Reddy CM (2015) Production of jet fuel range hydrocarbons as a coproduct of algal biodiesel by butenolysis of long-chain alkenones. Energy Fuels 29:922–930
Rybak A, Meier MAR (2007) Cross-metathesis of fatty acid derivatives with methyl acrylate: renewable raw materials for the chemical industry. Green Chem 9:1356–1361
Dixneuf PH, Bruneau C, Fischmeister C (2016) Alkene metathesis catalysis: a key for transformations of unsaturated plant oils and renewable derivatives. Oil Gas Sci Technol Rev 71:19
Griffin WC (1949) Classification of surface-active agents by HLB. J Soc Cosmet Chem 1:311–326
Maag H (1984) Fatty acid derivatives: important surfactants for household, cosmetic and industrial purposes. J Am Oil Chem Soc 61:259–267
Montero de Espinosa L, Meier MAR (2011) Plant oils: the perfect renewable resource for polymer science? Eur Polym J 47:837–852
Gary NE (1962) Chemical mating attractants in the queen honey bee. Science 136:773–774
Koeniger N, Koeniger G (2000) Reproductive isolation among species of the genus Apis. Apidologie 31:313–319
Nagaraja N, Brockmann A (2009) Drones of the dwarf honey bee Apis florea are attracted to (2E)-9-oxodecenoic acid and (2E)-10-hydroxydecenoic acid. J Chem Ecol 35:653–655
Winston ML, Slessor KN (1993) Applications of queen honey bee mandibular pheromone for beekee** and crop pollination. Bee World 74:111–128
O’Neil GW, Williams JR, Wilson-Peltier J, Knothe G, Reddy CM (2016) Experimental protocol for biodiesel production with isolation of alkenones as coproducts from commercial Isochrysis algal biomass. J Vis Exp 112:e54189
Conte MH, Thompson A, Lesley D, Harris RP (1998) Genetic and physiological influences on the alkenone/alkenoate versus growth temperature relationship in Emiliania huxleyi and Gephyrocapsa oceanica. Geochim Cosmochim Acta 62:51–68
Brassell SC, Eglinton G, Marlowe IT, Pflaumann U, Sarnthein M (1986) Molecular stratigraphy: a new tool for climatic assessment. Nature 320:129–133
Marlowe IT, Brassell SC, Eglinton G, Green JC (1984) Long chain unsaturated ketones and esters in living algae and marine sediments. Org Geochem 6:135–141
Prahl FG, Wakeham SG (1987) Calibration of unsaturation patterns in long-chain ketone compositions for palaeotemperature assessment. Nature 330:367–369
Eglinton G, Bradshaw SA, Rosell A, Sarnthein M, Pflaumann U, Tiedemann R (1992) Molecular record of secular sea surface temperature changes on 100-year timescales for glacial terminations I, II and IV. Nature 356:423–426
Müller PJ, Kirst G, Ruhland G, von Storch I, Rosell-Melé A (1998) Calibration of the alkenone paleotemperature index U37 K′ based on core-tops from the eastern South Atlantic and the global ocean (60°N–60°S). Geochim Cosmochim Acta 62:1757–1772
Volkman JK, Barrerr SM, Blackburn SI, Sikes EL (1995) Alkenones in Gephyrocapsa oceanica: implications for studies of paleoclimate. Geochim Cosmochim Acta 59:513–520
World Weather Online (2016) Olhão monthly climate average. https://us.worldweatheronline.com/v2/weather-averages.aspx?locid=2005567&root_id=1996459&wc=local_weather&map=~/olhao-weather-averages/faro/pt.aspx. Accessed 10 Nov 2016
Trzaskowski J, Quinzler D, Bährle C, Mecking S (2011) Aliphatic long-chain C20 polyesters from olefin metathesis. Macromol Rapid Commun 32:1352–1356
Reddy CM, Eglinton TI, Hounshell A, White H, Li Xu, Gaines RB, Frysinger GS (2002) The West Falmouth oil spill after thirty years: the persistence of petroleum hydrocarbons in marsh sediments. Environ Sci Technol 36:4754–4760
O’Neil GW, Nelson RK, Wright AM, Reddy CM (2016) A one-pot/single-analysis approach to substrate scope investigations using comprehensive two-dimensional gas chromatography (GC × GC). J Org Chem 81:3533–3541
Gros J, Nabi D, Wu B, Wick LY, Brussard CPD, Huisman J, van der Meer JR, Reddy CM, Arey JS (2014) First day of an oil spill on the open sea: early mass transfers of hydrocarbons to air and water. Environ Sci Technol 48:9400–9411
Labana SS (1997) Chemistry and properties of cross-linked polymers. Academic, New York
Hong SH, Day MW, Grubbs RH (2004) Decomposition of a key intermediate in ruthenium-catalyzed olefin metathesis reactions. J Am Chem Soc 126:7414–7415
Jenkins RW, Sargeant LA, Whiffin FM, Santomauro F, Kaloudis D, Mozzanega P, Bannister CD, Baena S, Chuck CJ (2015) Cross-metathesis of microbial oils for the production of advanced biofuels and chemicals. ACS Sustainable Chem Eng 3:1526–1535
Jida M, Betti C, Schiller SW, Tourwé D, Ballet S (2014) One-pot isomerization-cross metathesis–reduction (ICMR) synthesis of lipophilic tetrapeptides. ACS Comb Sci 16:342–351
Schmidt B, Hauke S (2013) Cross metathesis of allyl alcohols: how to suppress and how to promote double bond isomerization. Org Biomol Chem 11:4194–4206
Hong SH, Sanders DP, Lee CW, Grubbs RH (2005) Prevention of undesirable Isomerization during olefin metathesis. J Am Chem Soc 127:17160–17161
Patel J, Elaridi J, Jackson WR, Robinson AJ, Serelis AK, Such C (2005) Cross-metathesis of unsaturated natural oils with 2-butene. High conversion and productive catalyst turnovers. Chem Commun 2005:5546–5547
Chatterjee AK, Choi T-L, Sanders DP, Grubbs RH (2003) A general model for selectivity in olefin cross-metathesis. J Am Chem Soc 125:11360–11370
Segev E, Castañeda IS, Sikes EL, Vlamakis H, Kolter R (2016) Bacterial influence on alkenones in live microalgae. J Phycol 52:125–130
Acknowledgements
This work was supported by the National Science Foundation (CHE-1151492) and through a private donation from friends of WHOI.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
About this article
Cite this article
O’Neil, G.W., Williams, J.R., Craig, A.M. et al. Accessing Monomers, Surfactants, and the Queen Bee Substance by Acrylate Cross-Metathesis of Long-Chain Alkenones. J Am Oil Chem Soc 94, 831–840 (2017). https://doi.org/10.1007/s11746-017-2997-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-017-2997-8