Abstract
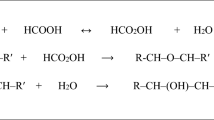
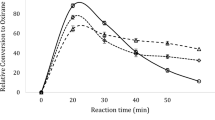
Epoxidation of karanja oil (KO), a nondrying vegetable oil, was carried out with peroxyacetic acid that was generated in situ from aqueous hydrogen peroxide and glacial acetic acid. KO contained 61.65% oleic acid and 18.52% linoleic acid, respectively, and had an iodine value of 89 g/100 g. Unsaturated bonds in the oil were converted to oxirane by epoxidation. Almost complete epoxidation of ethylenic unsaturation was achieved. For example, the iodine value of the oil could be reduced from 89 to 19 by epoxidation at 30°C. The effects of temperature, hydrogen peroxide-to-ethylenic unsaturation ratio, acetic acid-to-ethylenic unsaturation ratio, and stirring speed on the epoxidation rate and on oxirane ring stability were studied. The rate constant and activation energy for epoxidation of KO were 10−6 L·mol−1·s−1 and 14.9 kcal·mol−1, respectively. Enthalpy, entropy, and free energy of activation were 14.2 kcal·mol−1, −51.2 cal·mol−1·K−1, and 31.1 kcal·mol−1, respectively. The present study revealed that epoxides can be developed from locally available natural renewable resources such as KO.
Similar content being viewed by others
References
Adhvaryu, A., and S.Z. Erhan, Epoxidized Soybean Oil as a Potential Source of High Temperature Lubricants, Ind. Crops Prod. 15:247–254 (2002).
Blayo, A., A. Gandini, and J.F. Le Nest, Chemical and Rheological Characterizations of Some Vegetable Oil Derivatives Commonly Used in Printing Inks, 14:155–167 (2001).
Biermann, U., W. Friedt, S. Lang, W. Lühs, G. Machmüller, J.O. Metzger, M. Rüsch gen Klaas, H.J. Schäfer, and M.P. Schneider, New Syntheses with Oils and Fats as Renewable Raw Materials for the Chemical Industry, Angew. Chem. Int. Ed. 39:2206–2224 (2000).
Okieimen, F.E., O.I. Bakare, and C.O. Okieimen, Studies on the Epoxidation of Rubber Seed Oil, Ind. Crops Prod. 15: 139–144 (2002).
Warwel, S., and M. Rüsch gen Klaas, Complete and Partial Epoxidation of Plant Oils by Lipase-Catalyzed Perhydrolysis, 9:125–132 (1999).
Guenter, S., R. Rieth, and K.T. Rowbottom, Ullmann's Encyclopedia of Industrial Chemistry, 6th edn., John Wiley & Sons, 2003, Vol. 12, pp. 269–284.
Warwel, S., and M. Rüsch gen. Klaas, Chemo-enzymatic Epoxidation of Unsaturated Carboxylic Acids, J. Mol. Cat. B: Enzym. 1:29–35 (1995).
Sharpless, K.B., S.S. Woodard, and M.G. Finn, On the Mechanism of Titanium-Tartrate Catalyzed Asymmetric Epoxidation, Pure Appl. Chem. 55:1823–1836 (1983).
Sheldon, R.A., and J. Dakka, Heterogeneous Catalytic Oxidations in the Manufacture of Fine Chemicals, Catal. Today 19:215–246 (1994).
Campanella, A., M.A. Baltanas, M.C. Capel-Sanchez, J.M. Campos-Martin, and J.L.G. Fierro, Soybean Oil Epoxidation with Hydrogen Peroxide Using an Amorphous Ti/SiO2 Catalyst, Green Chem. 6:330–334 (2004).
Angelini, L.G., E. Moscheni, G. Colonna, P. Belloni, and E. Bonari, Variation in Agronomic Characteristics and Seed Oil Composition of New Oilseed Crops in Central Italy, Ind. Crops Prod. 6:313–323 (1997).
Warwel, S., F. Brüse, C. Demes, and M. Kunz, Polymers and Polymer Building Blocks from Meadowfoam Oil, 20:301–309 (2004).
Swern, D., Organic Peroxides, Chem. Rev. 45:1–521 (1949).
Paquot, C., Standard Methods for the Analysis of Oils, Fats and Derivatives Part 1, 6th edn., Pergamon, Oxford, United Kingdom, 1979, pp. 66–70.
May, C.A., Epoxy Resins: Chemistry and Technology, Marcel Dekker, New York, 1973, pp. 672–673.
Petrovic, Z.S., A. Zlatanic, C.C. Lava, and S. Sinadinovic-Fiser, Epoxidation of Soybean Oil in Toluene with Peroxyacetic and Peroxyformic Acids—Kinetics and Side Reactions, Eur. J. Lipid Sci. Technol. 104:293–299 (2002).
Goud, V.V., A.V. Patwardhan, and N.C. Pradhan, Epoxidation of Mahua Oil by Hydrogen Peroxide: Kinetic Study, Proceedings of 1st National Conference of Research Scholars and Young Scientists in Chemical Engineering, Chemical Engineering Department (I.I.T. Kharagpur, India), and I.I.Ch.E. (Kharagpur Regional Centre), Kharagpur, India, September 2004.
Gan, L.H., S.H. Goh, and K.S. Ooi, Kinetics Studies of Epoxidation and Oxirane Cleavage of Palm Olein Methyl Esters, J. Am. Oil Chem. Soc. 69:347–351 (1992).
Zaher, F.A., M.H. El-Mallah, and M.M. El-Hefnawy, Kinetics of Oxirane Cleavages in Epoxidized Soybean Oil, J. Am. Oil Chem. Soc. 66:698–700 (1989).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Goud, V.V., Pradhan, N.C. & Patwardhan, A.V. Epoxidation of karanja (Pongamia glabra) oil by H2O2 . J Amer Oil Chem Soc 83, 635–640 (2006). https://doi.org/10.1007/s11746-006-1250-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-006-1250-7