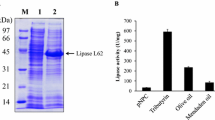
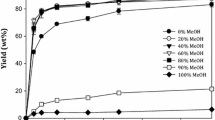
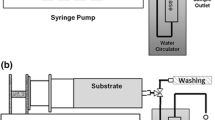
Abstract
Lipase from Pseudomonas fluorescens efficiently catalyzed the alcoholysis of various TG in dry alcohols. For TG with short-chain FA, more MG were accumulated. The yields of MG were affected by the alcohols used. The maximum yields of MG were as follows: 85% for monoacetin in n-butanol, 96% for monobutyrin in ethanol or n-butanol, 50% for monocaprylin in n-butanol, 48% for monolaurin in isopropanol, and 45% for monopalmitin in isopropanol. The MG produced were judged to be 2-MG by TLC analysis. The presence of organic cosolvent affected the reaction rate of the lipase-catalyzed alcoholysis of TG. For the alcoholysis of various TG in ethanol and cosolvent (1∶1, vol/vol), the rates had the following orders: (i) for tributyrin, hexane > toluene > acetone > ethyl acetate > chloroform > acetonitrile > pyridine; (ii) for tricaprylin, hexane > acetone > toluene > acetonitrile > ethyl acetate > pyridine > chloroform; and (iii) for trialurin, hexane > acetonitrile=acetone > ethyl acetate > pyridine=chloroform > toluene.
Similar content being viewed by others
References
Arcos, J.A., and C. Otero, Enzyme, Medium, and Reaction Engineering to Design a Low-Cost, Selective Production Method for Mono- and Dioleoylglycerols, J. Am. Oil Chem. Soc. 73:673–682 (1996).
Bornscheuer, U.T., Lipase-Catalyzed Syntheses of Monoacylglycerols, Enzyme Microb. Technol. 17:578–586 (1995).
Stutz, R.L., A.J. del Vecchio, R.J. Tenney, and C.J. Patterson, The Role of Emulsifiers and Dough Conditioners in Foods, Food Prod. Dev. 7:52–60 (1973).
Elfman Borjesson, I., and M. Harrod, Synthesis of Monoglycerides by Glycerolysis of Rapeseed Oil Using Immobilized Lipase, J. Am. Oil Chem. Soc. 76:701–707 (1999).
Fan, H.L., Y. Chu, G.X. Yang, W. Zhang, J.L. Liu, Z.S. Wu, S.G. Cao, and D.L. You, Lipase-Catalyzed Syntheses of Monoglycerides by Hydrolysis of Soybean Oil in AOT/Isooctane Reversed Micelles, Ann. N.Y. Acad. Sci. 864:267–272 (1998).
Hoq, M.M., T. Yamane, S. Shimizu, T. Funada, and S. Ishida, Continuous Synthesis of Glycerides by Lipase in a Microporous Membrane Bioreactor, J. Am. Oil Chem. Soc. 61:776–781 (1984).
Langone, M.A., M.E. De Abreu, M.J. Rezende, and G.L. Sant-Anna, Jr., Enzymatic Synthesis of Medium Chain Monoglycerides in a Solvent-Free System, Appl. Biochem. Biotechnol. 98–100:987–996 (2002).
Millqvist, A., P. Adlercreutz, and B. Mattiasson, Lipase-Catalyzed Alcoholysis of Triglycerides for the Preparation of 2-Monoglycerides, Enzyme Microb. Technol. 16:1042–1047 (1994).
Irimescu, R., K. Furihata, K. Hata, Y. Iwasaki, and T. Yamane, Utilization of Reaction Medium-Dependent Regiospecificity of Candida antarctica Lipase (Novozym 435) for the Synthesis of 1,3-Dicapryloyl-2-docosahexaenoyl (or eicosapentaenoyl) Glycerol, J. Am. Oil Chem. Soc. 78:285–289 (2001).
Irimescu, R., K. Furihata, K. Hata, Y. Iwasaki, and T. Yamane, Two-Step Enzymatic Synthesis of DHA-rich Symmetrically Structured Triacylglycerols via 2-Monoacylglycerols, Ibid., 78:473–478 (2001).
Shaw, J.F., and E.T. Liaw, Preparation of Acyl Derivatives of 1-Hydroxy Aldose by Lipase-Catalyzed Hydrolysis of Alcoholysis of Fully Acylated Aldose in Organic Solvent, in Biocatalysis in Organic Media, edited by C. Laane, J. Tramper, and M.D. Lilly, Elsevier, Amsterdam, 1987, pp. 233–239.
Shaw, J.F., D.L. Wang, and Y.J. Wang, Lipase-Catalysed Ethanolysis and Isopropanolysis of Triglycerides with Long-Chain Fatty Acids, Enzyme Microb. Technol. 13:544–546 (1991).
Dossat, V., D. Combes, and A. Marty, Efficient Lipase Catalysed Production of a Lubricant and Surfactant Formulation Using a Continuous Solvent-Free Process, J. Biotechnol. 97:117–124 (2002).
Vacek, M., M. Zarevucka, Z. Wimmer, K. Stransky, M. Mackova, and K. Demnerova, Enzymatic Alcoholysis of Blackcurrant Oil, Biotechnol. Lett. 23:27–32 (2001).
Coffen, D.L., Enzyme-Catalyzed Reactions, in Chiral Separations: Applications and Technology, edited by S. Ahuja, American Chemical Society, Washington, DC, 1997, pp. 59–91.
Gupta, R., N. Gupta, and P. Rathi, Bacterial Lipases: An Overview of Production, Purification and Biochemical Properties, Appl. Microbiol. Biotechnol. DOI: 10.1007/s00253-004-1568-8 (2004).
Rogalska, E., C. Cudrey, F. Ferrato, and R. Verger, Stereoselective Hydrolysis of Triglycerides by Animal and Microbial Lipases, Chirality 5:24–30 (1993).
Sugihara, Y. Shimada, and Y. Tominaga, A Novel Geotrichum candidum Lipase with Some Preference for the 2-Position on a Triglyceride Molecule, Appl. Microbiol. Biotechnol. 35:738–740 (1991).
Brobst, K.M., and C.E. Lott, Determination of Some Carbohydrates in Corn Syrups by Gas Chromatography of Trimethylsilyl Derivatives, Cereal Chem. 43:35–42 (1966).
Laane, C., S. Boeren, R. Hilhorst, and C. Veeger, Optimization of Biocatalysis in Organic Media, in Biocatalysis in Organic Media, edited by C. Laane, J. Tramper, and M. D. Lilly, Elsevier, Amsterdam, 1987, pp. 65–84.
Chang, R.C., S.J. Chou, and J.F. Shaw, Multiple Forms and Functions of Candida rugosa Lipase, Biotechnol. Appl. Biochem. 19:93–97 (1994).
Barton, M.J., J.P. Hamman, K.C. Fichter, and G.J. Calton, Enzymatic Resolution of (R,S)-2-(4-hydroxyphenoxy) Propionic Acid, Enzyme Microb. Technol. 12:577–583 (1990).
Shaw, J.F., C.H. Chang, and Y.J. Wang, Characterization of Three Distinct Forms of Lipolytic Enzyme in a Commercial Candida Lipase Preparation, Biotechnol. Lett. 11:779–784 (1989).
Lee, G.C., S.J. Tang, K.H. Sun, and J.F. Shaw, Analysis of the Gene Family Encoding Lipases in Candida rugosa by Competitive Reverse Transcription-PCR, Appl. Environ. Microbiol. 65:3888–3895 (1999).
Shaw, J.F., R.C. Chang, F.F. Wang, and Y.J. Wang, Lipolytic Activities of a Lipase Immobilized on Six Selected Supporting Materials, Biotechnol. Bioeng. 35:132–137 (1990).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lee, GC., Wang, DL., Ho, YF. et al. Lipase-catalyzed alcoholysis of triglycerides for short-chain monoglyceride production. J Amer Oil Chem Soc 81, 533–536 (2004). https://doi.org/10.1007/s11746-006-0936-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-006-0936-1