Abstract
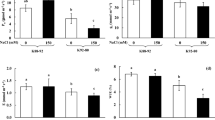
Plants adopt several strategies to maintain cellular ion homeostasis, including physiological, biochemical, cellular, subcellular, and molecular mechanisms for fighting against salt stress. We investigated the responses of tolerant Tibetan wild barley (XZ16), tolerant (CM72) and sensitive (Gairdner) barley cultivars at physiological, cellular, and molecular levels. The results revealed that salinity induced a significantly greater reduction in total root length, surface area, diameter, and total volume in Gairdner than in CM72 and XZ16. Analysis of gene expression using quantitative RT-PCR showed that transcripts of vacuolar H+-ATPase and inorganic pyrophosphatase (HvHVA/68 and HvHVP1) were more abundant in leaves and roots of XZ16 and CM72 than those of Gairdner. Observation of electron microscopy detected the difference in the damage of leaf and root ultrastructure among the three genotypes under salt stress, with XZ16 and Gairdner being least and most affected, respectively. Subcellular study showed that a primary strategy to protect the cytosol against sodium toxicity was compartmentalization of sodium ions into soluble fraction (vacuoles). Gairdner showed drastically stronger sodium-specific fluorescence visualized by CoroNa-Green, a sodium-specific fluorophore, than CM72 and XZ16.








Similar content being viewed by others
Abbreviations
- SPB:
-
Sodium phosphate buffer
- TEM:
-
Transmission electron microscopy
- ICP–OES:
-
Inductively coupled plasma–optical emission spectrometry
References
Apse MP, Blumwald E (2007) Na+ transport in plants. FEBS Lett 581:2247–2254
Apse MP, Aharon GS, Sneddon WA, Blumwald E (1999) Salt tolerance conferred by over expression of a vacuolar Na+/H+ antiport in Arabidopsis. Sci 285:1256–1258
Ayala F, Leary JW, Schumaker KS (1996) Increased vacuolar and plasma membrane H+-ATPase activities in Salicornia bigelovii Torr in response to NaCl. J Exp Bot 47:25–32
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465:140–151
Brini F, Hanin M, Mezghani I, Berkowitz GA, Masmoudi K (2007) Overexpression of wheat Na+/H+ antiporter NHX1 and H+- pyrophosphatase TVP1 improve salt- and drought-stress tolerance in Arabidopsis thaliana plants. J Exp Bot 58:301–308
Dong Hh, Eduardo L, Quan Z, Sung-M H, Youzhi L, Francisco JQ, **ngyu J, Matilde PDU, Sang YL, Yanxiu Z, Jeong DB, Ray AB, Dae JY, Jose MP, Hans JB (2009) Loss of halophytism by interference with SOS1 expression. Plant Physiol 151:210–222
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55(396):307–319
Flowers TJ, Colmer TO (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Flowers TJ, Hajibagheri MA (2001) Salinity tolerance in Hordeum vulgare ion concentrations in root cells of cultivars differing in salt tolerance. Plant Soil 231:1
Fukuda A, Nakamura A, Tagiri A, Tanaka H, Miyao A, Hirochika H, Tanaka Y (2004) Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol 45:146–159
Gao F, Gao Q, Duan XG, Yue GD, Yang AF, Zhang JR (2006) Cloning of an H+-PPase gene from Thellungiella halophila and its heterologous expression to improve tobacco salt tolerance. J Exp Bot 57:3259–3270
Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581:2204–2214
Golldack D, Dietz KJ (2001) Salt-induced expression of the vacuolar H+-ATPase in the common ice plant is developmentally controlled and tissue specific. Plant Physiol 125:1643–1654
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
He XL, Huan X, Shen YZ, Huang ZJ (2014) Wheat V-H+-ATPase subunit genes significantly affect salt tolerance in Arabidopsis thaliana. PLoS ONE 9(8):e86982
Kohei H, Megumi N, Katsuhisa Y, Miwa O, Yoshihisa O, Tomohiro U, Go Tatsuaki, Masa HS, Miyo TM, Masao T, Sei-ichiro HAN, Ikuko HN, Masayoshi M, Hidehiro F, Tetsuro M (2009) Dynamic aspects of ion accumulation by vesicle traffic under salt stress in Arabidopsis. Plant Cell Physiol 50(12):2023–2033
Krebs M, Beyhl D, Gorlich E, Al-Rasheid AS, Marten I, Stierhof YD, Hedrich R, Schumacher K (2010) Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc Natl Acad Sci USA 107:3251–3256
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, San Diego
Maser P, Gierth M, Schroeder JI (2002) Molecular mechanisms of potassium and sodium uptake in plants. Plant Soil 247:43–54
Mehea P, Hyosuk L, Jung SL, Ok Myung, Beom-Gi B, Kim MP (2009) In planta measurements of Na+ using fluorescent dye CoroNa Green. J Plant Biol 52:298–302
Mitsuya S, Takeoka Y, Miyake H (2000) Effects of sodium chloride on foliar ultrastructure of sweet potato (Ipomoea batatas Lam) plantlets grown under light and dark conditions in vitro. J Plant Physiol 157:661–667
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Physiol 59:651–681
Okamura H, Aoyama I (1994) Interactive toxic effect and distribution of heavy metals in phytoplankton. Environ Toxicol Water Qual 9:7–15
Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE (2007) Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci USA 104:15619–15624
Parida AK, Das AB (2004) Effects of NaCl stress on nitrogen and phosphorus metabolism in a true mangrove Bruguiera parviflora grown under hydroponic culture. J Plant Physiol 161:921–928
Peng HY, Yang XE, Tian SK (2005) Accumulation and ultrastructural distribution of copper in Elasholtzia splendens. J Zhejiang Univ Sci B 6:311–318
Qiu L, Wu DZ, Ali S, Cai SG, Dai F, Zhang GP (2011) Evaluation of salinity tolerance and analysis of allelic function of HvHKT1 and HvHKT2 in Tibetan wild barley. Theor Appl Genet 122:695–703
Shuang W, Tobias B, Kimberly LG (2012) Mechanical fixation techniques for processing and orienting delicate samples, such as the root of Arabidopsis thaliana, for light or electron microscopy. Nat Protoc 7:1113–1124
Suzuki A, Vergnet C, Morot-Gaudry JF, Zehnacker C, Grosclaude J (1994) Immunological characterization of ferredoxin and methyl viologen interaction domains of glutamate synthase using monoclonal antibodies. Plant Physiol Biochem 32:619–626
Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, Donald Mc (2011) Additive effects of Na+ and Cl− ions on barley growth under salinity stress. J Exp Bot 62:2189–2203
Tester M, Davenport RJ (2003) Na+ transport and Na+ tolerance in higher plants. Ann Bot 91:503–527
Vera-Estrella R, Barkla BJ, Garcia-Ramirez L, Pantoja O (2005) Salt stress in Thellungiella halophila activates Na+ transport mechanisms required for salinity tolerance. Plant Physiol 139:1507–1517
Wang XC, Chang LL, Wang BC, Wang D, Li PH, Wang LM, Yi XP, Huang QX, Peng M, Guo AP (2013) Comparative Proteomics of Thellungiella halophila leaves from plants subjected to salinity reveals the importance of chloroplastic starch and soluble sugars in halophyte salt tolerance. Mol Cell Proteomics 12:2174–2195
Wu DZ, Qiu L, Xu L, Ye LZ, Chen MX, Zhang GP (2011) Genetic variation of HvCBF genes and their association with salinity tolerance in Tibetan annual wild barley. PLoS One 6:e22938
Wu D, Cai SG, Chen M, Ye LZ, Chen ZG, Wu FB, Zhang GP (2013) Tissue metabolic responses to salt stress in wild and cultivated barley. PLoS One 8:e55431
**a FC, Jiang GQ, Lu JM (2002) Development of the study on Halophyte’s structure of resisting saline-alkali. J TongHua Teach Coll 23(2):67–69
Yamane K, Mitsuya S, Taniguchi W, Miyake H (2012) Salt-induced chloroplast protrusionis the process of exclusion of ribulose-1,5-bisphosphate carboxylase/oxygenase from chloroplasts into cytoplasm in leaves of rice. Plant Cell Environ 35:1663–1671
Zeng F, Zhou W, Qiu B, Ali S, Wu F, Zhang G (2011) Subcellular distribution and chemical forms of chromium in rice plants suffering from different levels of chromium toxicity. J Plant Nutr Soil Sci 174:249–256
Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768
Zhao J, Sun HY, Dai HX, Zhang GP, Wu FB (2010) Difference in response to drought stress among Tibet wild barley genotypes. Euphytica 172:395–403
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Acknowledgments
This research was supported by National Natural Science Foundation of China (No. 31330055, 31301246 and 31171544), China Agriculture Research System (CARS-05).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. V. Jorrin-Novo.
Rights and permissions
About this article
Cite this article
Jabeen, Z., Hussain, N., Han, Y. et al. The differences in physiological responses, ultrastructure changes, and Na+ subcellular distribution under salt stress among the barley genotypes differing in salt tolerance. Acta Physiol Plant 36, 2397–2407 (2014). https://doi.org/10.1007/s11738-014-1613-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1613-x