Abstract
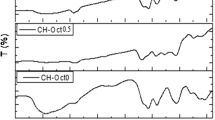
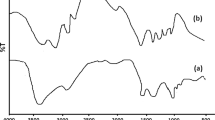
In this study, H-form oleoyl-carboxymethyl-chitosan (H-O-CMCS) was prepared as a coagulation agent to clean up the residual oil from the waste-water of oil extraction (WWOE). The Fourier transform infrared (FTIR) spectra confirmed the formation of an amide linkage between amino groups of carboxymethyl chitosan (CMCS) and carboxyl groups of oleic acid. The adsorption capacities of four absorbents (H-O-CMCS, chitosan, activated carbon and polyaluminium chloride (PAC)) for the residual oil were investigated. Compared with chitosan, activated carbon and PAC, H-O-CMCS was more effective in removing the residual oil from WWOE, which could successfully wash up almost 99% of residual oil from WWOE at the dosage of 0.2 g/L, the mixing time of 3 min, 500 rpm, and a broader range of pH (the system temperature ⩽ 45°C). In similar conditions, comparatively, chitosan, activated carbon and PAC could wash 90%, 82% and 92% of residual oil from WWOE, respectively.
Similar content being viewed by others
References
Arcadio P S, Gregoria A S. Physical-chemical Treatment of Water and Waste-water. Washington, D C: CRC Press, IWA Publishing, 2003
Zouboulis A I, Avranas A. Treatment of oil-in-water emulsions by a coagulation agent and dissolved-air flotation. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2000, 172(1–3): 153–161
Koh A, Gillies G, Gore J, et al. Flocculation and coalescence of oil-in-water poly(dimethylsiloxane) emulsions. Journal of Colloid and Interface Science, 2000, 227(2): 390–397
Menezes F M, Amal R, Luketina D. Removal of particles using a coagulation agent and flocculation in a dynamic separator. Powder Technology, 1996, 88(1): 27–31
Edzwald J K. Coagulation in drinking water treatment: particles, organics, and coagulants. Water Science and Technology, 1993, 27(11): 21–35
Al-Mutairi N Z, Hamoda M F, Al-Ghusain I. Coagulant selection and sludge conditioning in a slaughterhouse wastewater treatment plant. Bioresource Technology, 2004, 95(2): 115–119
McCurdy K, Carlson K, Gregory D. Floc morphology and cyclic shearing recovery: comparison of alum and polyaluminum chloride coagulants. Water Research, 2004, 38(2): 486–494
Pontius F W. Regulation for aluminium in drinking water. Journal of American Water Works Association, 2000, 92(4): 18–22
Babel S, Kurniawan T A. Low-cost adsorbents for heavy metals uptake from contaminated water: a review. Journal of Hazardous Materials, 2003, 97(1–3): 219–243
Ravi Kumar M N V. A review of chitin and chitosan applications. Reactive and Functional Polymers, 2000, 46(1): 1–27
An H K, Park B Y, Kim D S. Crab shell for the removal of heavy metals from aqueous solution. Water Research, 2001, 35(15): 3551–3556
Ahmad A L, Sumathi S, Hameed B H. Adsorption of residue oil from palm oil mill effluent using powder and flake chitosan: Equilibrium and kinetic studies. Water Research, 2005, 39(12): 2483–2494
Chen X-G, Park H-J. Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydrate Polymers, 2003, 53(4): 355–359
Sakairi N, Suzuki S, Ueno K, et al. Biosynthesis of heteropolysaccharides by Acetobacter xylinum-Synthesis and characterization of metal-ion adsorptive properties of partially carboxmethylated cellulose. Carbohydrate Polymers, 1998, 37(4): 409–414
Liu X F, Guan Y L, Yang D Z, et al. Antibacterial action of chitosan and carboxymethylated chitosan. Journal of Applied Polymer Science, 2001, 79(7): 1324–1335
Zhao X, Shang S H, Zhao P. Synthesis and properties of a modified chitosan spilled oil gelling agent on water. Chemical World, 2005, (10): 621–624 (in Chinese)
Shigemasa Y, Matsuura H, Sashiwa H, et al. Evaluation of different absorbance ratios from infrared spectroscopy for analyzing the degree of deacetylation in chitin. International Journal of Biological Macromolecules, 1996, 18(3): 237–242
Ahmad A L, Sumathi S, Hameed B H. Residual oil and suspended solid removal using natural adsorbents chitosan, bentonite and activated carbon: A comparative study. Chemical Engineering Journal, 2005, 108(1–2): 179–185
Chow M C, Ho C C. Surface active properties of palm oil with respect to the processing of palm oil. Journal of Palm Oil Research, 2000, 12(1): 107–116
Meyssami B, Kasaeian A B. Use of coagulants in treatment of olive oil wastewater model solutions by induced air flotation. Bioresource Technology, 2005, 96(3): 303–307
Ahmad A L, Ismail S, Ibrahim N, et al. Removal of suspended solid and residual oil from palm oil mill effluent. Journal of Chemical Technology & Biotechnology, 2003, 78(9): 971–978
American Public Health Association, American Water Works Association & Water Pollution Control Federation. Standard Methods for the Examination of Water and Waste-water, 18th ed. Washington, D C: APHA, 1992
Chen X-G, Lee C M, Park H-J. O/W emulsification for the self-aggregation and nanoparticle formation of linoleic acidsmodified chitosan in the aqueous system. Journal of Agricultural and Food Chemistry, 2003, 51(10): 3135–3139
Osman Z, Arof A K. FTIR studies of chitosan acetate based polymer electrolytes. Electrochimica Acta, 2003, 48(8): 993–999
Pan J R, Huang C, Chen S, et al. Evaluation of a modified chitosan biopolymer for a coagulation agent of colloidal particles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1999, 147(3): 359–364
Chiou M S, Li H Y. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere, 2003, 50(8): 1095–1105
bin Hussein M Z, Kuang D, Zainal Z, et al. Kaolin-carbon adsorbents for carotene removal of red palm oil. Journal of Colloid and Interface Science, 2001, 235(1): 93–100
Ahmad A L, Sumathi S, Hameed B H. A coagulation agent of residue oil and suspended solid in palm oil mill effluent by chitosan, alum and PAC. Chemical Engineering Journal, 2006, 118(1–2): 99–105
Divakaran R, Sivasankara Pillai V N. Flocculation of kaolinite suspensions in water by chitosan. Water Research, 2001, 35(16): 3904–3908
Schmuhl R, Krieg H M, Keizer K. Adsorption of Cu(II) and Cr(IV) ions bychitosan: kinetics and equilibrium studies. Water SA, 2001, 27(1): 1–8
Zhao Y Q. Correlations between floc physical properties and optimum polymer dosage in alum sludge conditioning and dewatering. Chemical Engineering Journal, 2003, 92(1–3): 227–235
Eilbeck W J, Mattock G. Chemical Processes in Waste-water Treatment. England: Ellis Horwood Limited, 1987
Guibal E. Interactions of metal ions with chitosan-based sorbents: a review. Separation and Purification Technology, 2004, 38(1): 43–74
Schulz P C, Rodriguez M S, Del Blanco L F, et al. Emulsification properties of chitosan. Colloid & Polymer Science, 1998, 276(12): 1159–1165
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Gz., Chen, Xg., Li, Yy. et al. Preparation of H-oleoyl-carboxymethyl-chitosan and the function as a coagulation agent for residual oil in aqueous system. Front. Mater. Sci. China 2, 105–112 (2008). https://doi.org/10.1007/s11706-008-0019-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-008-0019-3