Abstract
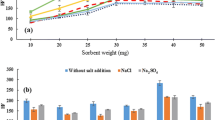
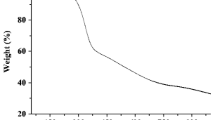
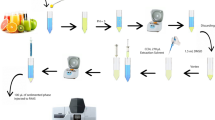
In this study, a simple, fast, green, and efficient extraction procedure based on a using of mixture of ibuprofen and choline chloride was developed to extract trace amounts of Cu(II) and Cd(II) ions from water and fruit juice samples. For this purpose, first, appropriate amounts of ibuprofen and choline chloride were mixed and after heating, a homogeneous liquid mixture was formed. The synthesized hydrophobic mixture was used to prepare an adsorbent for the extraction of the analytes from the samples. Then extracted analytes were enriched by a dispersive liquid–liquid microextraction method before their determination by flame atomic absorption spectrometry. To achieve the best possible results, the effect of various parameters on the extraction recoveries of the analytes was investigated. Under optimal experimental conditions, linear ranges of the calibration curves were in the ranges of 2.5–100 μg L−1 for Cd(II) and 0.50–75 μg L−1 for Cu(II). The obtained relative standard deviations (n = 6, C = 10 and 25 μg L−1 of each cation) were in the ranges of 2.5–4.2%. Limits of quantifications of 0.50 and 2.5 µg L−1 were obtained for Cu(II) and Cd(II), respectively. Finally, the proposed method was successfully used for the determination of Cu(II) and Cd(II) ions in various water and fruit juice samples by providing high extraction recoveries in the range of 94–97% for Cu(II) and Cd(II), respectively.









Similar content being viewed by others
References
Abed Altuwaijari HN, Farajzadeh MA, Afshar Mogaddam MR, Sorouraddin SM (2023) In-situ formation of a solid adsorbent for the extraction of some metal ions from crude oil before their determination by micro flow nebulizer inductively coupled plasma-mass spectrometry. Talanta 257:124378
Akl MA, AL-Rabasi A, Molouk AF (2021) Cloud point extraction and FAAS determination of copper (II) at trace level in environmental samples using N-benzamido-N’-benzoylthiocarbamide and CTAB. Egypt J Chem 64:313–322
Altunay N, Elik A, Gürkan R (2019) Monitoring of some trace metals in honeys by flame atomic absorption spectrometry after ultrasound assisted-dispersive liquid liquid microextraction using natural deep eutectic solvent. Microchem J 147:49
Chen B, Zhang L, He M, Hu B (2020) Cd(II) imprinted polymer modified silica monolithic capillary microextraction combined with inductively coupled plasma mass spectrometry for the determination of trace Cd (II) in biological samples. Spectrochim Acta B 164:105751
Chisvert A, Cárdenas S, Lucena R (2019) Dispersive micro-solid phase extraction. Trends Anal Chem 112:226
Cui C, Hu B, Chen B, He M (2013) Ionic liquid-based magnetic solid phase extraction coupled with inductively coupled plasma-optical emission spectrometry for the determination of Cu, Cd, and Zn in biological samples. J Anal Atom Spectrom 28:1110
Deng R, Huang D, Wan J, Xue W, Lei L, Wen X, Liu X, Chen S, Yang Y, Li Z, Li B (2019) Chloro-phosphate impregnated biochar prepared by co-precipitation for the lead, cadmium and copper synergic scavenging from aqueous solution. Bioresource Technol 293:122102
Ellingsen DG, Horn N, Aaseth J (2007) Handbook on the Toxicology of Metals. Elsevier
Farajzadeh MA, Sattari Dabbagh M (2020) Development of a dispersive solid phase extraction method based on in situ formation of adsorbent followed by dispersive liquid–liquid microextraction for extraction of some pesticide residues in fruit juice samples. J Chromatogr A 1627:461389
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J (2012) Analytical Eco-Scale for assessing the greenness of analytical procedures. Trends Anal Chem 37:61–72
Ghorbani M, Aghamohammadhassan M, Chamsaz M, Akhlaghi H, Pedramrad T (2019) Dispersive solid phase microextraction. Trends Anal Chem 118:793
Jabbari S, Sorouraddin SM, Farajzadeh MA, Fathi AA (2023) Determination of copper(II) and lead(II) ions in dairy products by an efficient and green method of heatinduced homogeneous liquid-liquid microextraction based on a deep eutectic solvent. Anal Methods 15:4321–4330
Karlıdağ NE, Demirel R, Serbest H, Turak F, Bakırdere S (2023) Determination of cobalt in chamomile tea samples at trace levels by flame atomic absorption spectrophotometry after poly(vinyl alcohol)-magnetic hydrogel based dispersive solid phase extraction. Anal Methods 15:56–62
Kazantzi V, Drosaki E, Skok A, Vishnikin AB, Anthemidis A (2019) Evaluation of polypropylene and polyethylene as sorbent packing materials in on-line preconcentration columns for trace Pb (II) and Cd (II) determination by FAAS. Microchem J 148:514
Khaghani S, Ezzatpanah H, Mazhari N, Givianrad MH, Mirmiranpour H, Sadrabadi FS (2010) Zinc and copper concentrations in human milk and infant formulas. Iran J Pediatr 20:53
Khezeli T, Daneshfar A (2017) Development of dispersive micro-solid phase extraction based on micro and nano sorbents. Trends Anal Chem 89:99
Khiat M, Pacheco-Fernández I, Pino V, Benabdallah T, Ayala JH, Afonso AM (2018) A guanidinium ionic liquid-based surfactant as an adequate solvent to separate and preconcentrate cadmium and copper in water using in situ dispersive liquid–liquid microextraction. Anal Method 10:1529–1537
Lee LY, Morad N, Ismail N, Talebi A, Rafatullah M (2020) Optimization for liquid-liquid extraction of Cd (Ii) over Cu (ii) ions from aqueous solutions using ionic liquid aliquat 336 with tributyl phosphate. Inter J Mol Sci 21:6860
Li X, Zhang Q, Yang B (2020) Co-precipitation with CaCO3 to remove heavy metals and significantly reduce the moisture content of filter residue. Chemosphere 239:124660
Liu C, Bi X, Zhang A, Qi B, Yan S (2020) Preparation of an L-cysteine functionalized magnetic nanosorbent for the sensitive quantification of heavy metal ions in food by graphite furnace atomic absorption spectrometry. Anal Lett 53:2079
Mane CP, Mahamuni SV, Kolekar SS, Han SH, Anuse MA (2016) Hexavalent hromium recovery by liquid–liquid extraction with 2-octylaminopyridine from acidic chloride media and its sequential separation from other heavy toxic metal ions. Arab J Chem 9:S1420
Manjusha R, Shekhar R, Kumar SJ (2019) Ultrasound-assisted extraction of Pb, Cd, Cr, Mn, Fe, Cu, Zn from edible oils with tetramethylammonium hydroxide and EDTA followed by determination using graphite furnace atomic absorption spectrometer. Food Chem 294:384
Marahel F, Ghaedi M, Montazerozohori M, Biyareh MN, Kokhdan SN, Soylak M (2011) Solid-phase extraction and determination of trace amount of some metal ions on Duolite XAD 761 modified with a new Schiff base as chelating agent in some food samples. Food Chem Toxicol 49:208
Moraes ML, Feijó M, Melo FM, Campos RC, Hauser-Davis RA (2009) Iron, copper and zinc in substitute foods for maternal milk: comparison with infant nutritional requirements. J Brazil Chem Soc 20:1724
Paktsevanidou IP, Manousi N, Zachariadis GA (2021) Development and validation of an inductively coupled plasma–atomic emission spectrometry (ICP-AES) method for trace element determination in vinegar. Anal Lett 54:2227
Plotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta 181:204–209
Pourmohammad M, Faraji M, Jafarinejad S (2020) Extraction of chromium (VI) in water samples by dispersive liquid-liquid microextraction based on deep eutectic solvent and determination by UV-Vis spectrophotometry. Int J Environ Anal Chem 100:1146–1159
Racheva PV, Milcheva NP, Genc F, Gavazov KB (2021) A centrifuge-less cloud point extraction-spectrophotometric determination of copper (II) using 6-hexyl-4-(2-thiazolylazo) resorcinol. Spectrochim Acta A 262:120106
Rehan I, Gondal MA, Almessiere MA, Dakheel RA, Rehan K, Sultana S, Dastageer MA (2021) Nutritional and toxic elemental analysis of dry fruits using laser induced breakdown spectroscopy (LIBS) and inductively coupled plasma atomic emission spectrometry (ICP-AES). Saudi J Biol Sci 28:408
Rohanifar A, Rodriguez LB, Devasurendra AM, Alipourasiabi N, Anderson JL, Kirchhoff JR (2018) Solid-phase microextraction of heavy metals in natural water with a polypyrrole/carbon nanotube/1, 10–phenanthroline composite sorbent material. Talanta 188:570
Sajid M, Khaled Nazal M, Ihsanullah I (2021) Novel materials for dispersive (micro) solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples: A review. Anal Chim Acta 1141:246
Saleem PH, Moinfar S, Abdulazeez Mohammed I (2023) Determination of Cr and Pb in edible vegetable oils by coupling of extraction induced by emulsion breaking with dispersive liquid-liquid microextraction followed by flame atomic absorption spectrometry detection. J Food Compos Anal 124:105683
Shahryari T, Singh P, Raizada P, Davidyants A, Thangavelu L, Sivamani S, Naseri A, Vahidipour F, Ivanets A, Hosseini-Bandegharaei A (2022) Adsorption properties of Danthron-impregnated carbon nanotubes and their usage for solid phase extraction of heavy metal ions. Colloid Surface A 641:128528
Sorouraddin SM, Farajzadeh MA, Hassanyani A, Afshar Mogaddam MR (2016) Combination of homogenous liquid-liquid extraction and dispersive liquid-liquid microextraction for extraction and preconcentration of amantadine from biological samples followed by its indirect determination by flame atomic absorption spectrometry. RSC Adv 6:108603
Sorouraddin SM, Farajzadeh MA, Dastoori H (2020) Development of a dispersive liquid-liquid microextraction method based on a ternary deep eutectic solvent as chelating agent and extraction solvent for preconcentration of heavy metals from milk samples. Talanta 208:120485
Suo L, Dong X, Gao X, Xu J, Huang Z, Ye J, Lu X, Zhao L (2019) Silica-coated magnetic graphene oxide nanocomposite based magnetic solid phase extraction of trace amounts of heavy metals in water samples prior to determination by inductively coupled plasma mass spectrometry. Microchem J 149:104039
Tobiszewski M (2016) Metrics for green analytical chemistry. Anal Methods 8:2993–2999
Vajedi F, Dehghani H (2019) The characterization of TiO2-reduced graphene oxide nanocomposites and their performance in electrochemical determination for removing heavy metals ions of cadmium (II), lead (II) and copper (II). Mater Sci Eng B 243:189
Wang Y, Zhao H, Fei D, Shao Y, Liu J, Jiang G, **ng M (2019) Discrepant effects of copper (II) stress on different types of skeletal muscles in chicken: elements and amino acids. Ecotox Environ Safe 167:227
Wen X, Yang Q, Yan Zh, Deng Q (2011) Determination of cadmium and copper in water and food samples by dispersive liquid-liquid microextraction combined with UV–vis spectrophotometry. Microchem J 97:249–254
World Health Organization (1996) Trace elements in human nutrition and health. World Health Organization.
Wu G, Kang H, Zhang X, Shao H, Chu L, Ruan C (2010) A critical review on the bio-removal of hazardous heavy metals from contaminated soils: issues, progress, eco-environmental concerns and opportunities. J Hazard Mater 174:1
Wu W, Jia M, Zhang Z, Chen X, Zhang Q, Zhang W, Li P, Chen L (2019) Sensitive, selective and simultaneous electrochemical detection of multiple heavy metals in environment and food using a low cost Fe3O4 nanoparticles/fluorinated multi-walled carbon nanotubes sensor. Ecotox Environ Safe 175:243
Xu C, Peng C, He M, Chen B, Hu B (2021) Magnetic N-doped porous carbon for analysis of trace Pb and Cd in environmental water by magnetic solid phase extraction with inductively coupled plasma mass spectrometry. Spectrochim Acta B 184:106273
Yalçınkaya Ö, Kalfa OM, Türker AR (2011) Chelating agent free-solid phase extraction (CAF-SPE) of Co (II), Cu (II) and Cd (II) by new nano hybrid material (ZrO2/B2O3). J Hazard Mater 195:332
Yilmaz E, Soylak M (2014) Solid phase extraction of Cd, Pb, Ni, Cu, and Zn in environmental samples on multiwalled carbon nanotubes. Environ Monit Assess 186:5461
Zou J, Ma X, Dang Y, Chen Y (2014) Trace determination of cadmium (II) and copper (II) in environmental water samples by solid-phase extraction using a novel ionic liquid-modified composite sorbent combined with flame atomic absorption spectrometry. J Anal Atom Spectrom 29:1692
Zounr RA, Tuzen M, Deligonul N, Khuhawar MY (2018) A highly selective and sensitive ultrasonic assisted dispersive liquid phase microextraction based on deep eutectic solvent for determination of cadmium in food and water samples prior to electrothermal atomic absorption spectrometry. Food Chem 253:277
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shakourzadeh, Z., Sorouraddin, S.M., Farajzadeh, M.A. et al. In situ formation of an adsorbent using a mixture of ibuprofen and choline chloride for the extraction of Cu(II) and Cd(II) ions from water and fruit juice samples combined with dispersive liquid–liquid microextraction. Chem. Pap. 78, 5501–5512 (2024). https://doi.org/10.1007/s11696-024-03491-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-024-03491-6